Aromatase: Difference between revisions
imported>David E. Volk (chem diagram added) |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
{{Image|Aromatase CytP450 with androstenedione.png|right|350px|Human placental aromatase bound to ligands [[androstenedione]] (cyan) and [[heme]] (yellow). Coordinates obtained from the RCSB Protein Data Bank (3EQM).}} | {{Image|Aromatase CytP450 with androstenedione.png|right|350px|Human placental aromatase bound to ligands [[androstenedione]] (cyan) and [[heme]] (yellow). Coordinates obtained from the RCSB Protein Data Bank (3EQM).}} | ||
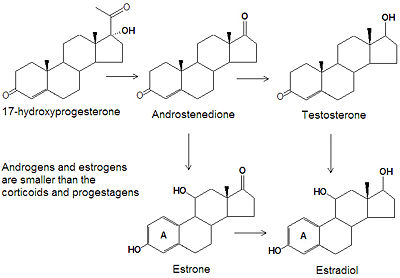
'''Aromatase''' is an [[enzyme]] that removes the C-19 methyl group and oxidizes the 3- and 17-positions of androgenic [[steroid]]s to convert them into estrogenic steroids (estrogen). It oonverts androstenedione, testosterone and 16-hydroxytestosterone into estrogen, 17<math>\beta</math>-estradiol and 17<math>\beta</math>-,16<math>\alpha</math>--estriol, respectively.<ref>{{cite journal|authors=EA Thompson & PK Siiteri|title=Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione|journal=J. Biol. Chem.|volume=249|pages=5364-5372|year=1974}}</ref>, | '''Aromatase''', the 503-amino acid long [[protein]] product of the ''CYP19A1'' [[gene]] on [[chromosome]] 15q21.1, is an [[enzyme]] that removes the C-19 methyl group via two oxidation steps and oxidizes the 3- and 17-positions of androgenic [[steroid]]s to convert them into estrogenic steroids (estrogen). It oonverts androstenedione, testosterone and 16<math>\alpha</math>-hydroxytestosterone into estrogen, 17<math>\beta</math>-estradiol and 17<math>\beta</math>-,16<math>\alpha</math>--estriol, respectively.<ref>{{cite journal|authors=EA Thompson & PK Siiteri|title=Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione|journal=J. Biol. Chem.|volume=249|pages=5364-5372|year=1974}}</ref>, | ||
<ref>{{cite journal|authors=ER Simpson et al.|title=Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis|journal=Endocr. Rev|volume=15|pages=342-355|year=1994}}</ref>, | <ref>{{cite journal|authors=ER Simpson et al.|title=Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis|journal=Endocr. Rev|volume=15|pages=342-355|year=1994}}</ref>, | ||
<ref>{{cite journal|authors=O'Neal & Johnston|title=Aromatase inhibitors|journal= Crit. Rev. Biochem. Mol. Biol.|volume=33|pages=375-405|year=1998}}</ref> Because some tumors are estrogen-sensitive, [[aromatase inhibitor]]s are sometimes used to treat cancer, mostly in post-menopausal women because aromatase inhibitors do not block estrogen synthesis in the ovaries. | <ref>{{cite journal|authors=O'Neal & Johnston|title=Aromatase inhibitors|journal= Crit. Rev. Biochem. Mol. Biol.|volume=33|pages=375-405|year=1998}}</ref> Because some tumors are estrogen-sensitive, [[aromatase inhibitor]]s are sometimes used to treat cancer, mostly in post-menopausal women because aromatase inhibitors do not block estrogen synthesis in the ovaries. | ||
| Line 16: | Line 16: | ||
== References == | == References == | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 13 July 2024
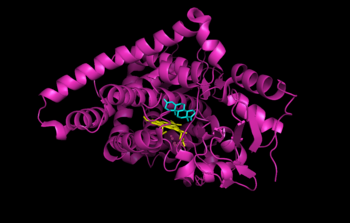
Human placental aromatase bound to ligands androstenedione (cyan) and heme (yellow). Coordinates obtained from the RCSB Protein Data Bank (3EQM).
Aromatase, the 503-amino acid long protein product of the CYP19A1 gene on chromosome 15q21.1, is an enzyme that removes the C-19 methyl group via two oxidation steps and oxidizes the 3- and 17-positions of androgenic steroids to convert them into estrogenic steroids (estrogen). It oonverts androstenedione, testosterone and 16-hydroxytestosterone into estrogen, 17-estradiol and 17-,16--estriol, respectively.[1], [2], [3] Because some tumors are estrogen-sensitive, aromatase inhibitors are sometimes used to treat cancer, mostly in post-menopausal women because aromatase inhibitors do not block estrogen synthesis in the ovaries.
Structure and function
Aromatase is the only enzyme in vertibrates that is capable of synthesizing all of the estrogen steroids from androgens, and has therefore been of great interest as a treatment for estrogen-sensitive tumors. Despite decades of research into aromatase, its structure was only determined in 2009. The structure of human placental aromatase cytochrome P450 bound to ligands androstenedione (cyan) and heme (yellow) was determined by x-ray crystallography.[4]
References
- ↑ (1974) "Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione". J. Biol. Chem. 249: 5364-5372.
- ↑ (1994) "Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis". Endocr. Rev 15: 342-355.
- ↑ (1998) "Aromatase inhibitors". Crit. Rev. Biochem. Mol. Biol. 33: 375-405.
- ↑ (January 8) "Structural Basis for Androgen Specificity and Estrogen Synthesis in Human Aromatase". Nature 457: 219-225. DOI:10.1038/nature07614. Research Blogging.