Hydrocarbons: Difference between revisions
imported>Milton Beychok |
mNo edit summary |
||
| (20 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
'''Hydrocarbons''' are a class of [[molecule]]s that contain only [[carbon]] and [[hydrogen]] atoms. Some of them make very good fuels. Gasoline contains a mixture of hydrocarbons. Unsaturated hydrocarbons, which contain one or more double bonds, are useful chemicals for many reactions. | '''Hydrocarbons''' are a class of [[molecule]]s that contain only [[carbon]] and [[hydrogen]] atoms. Some of them make very good fuels. [[Gasoline]] contains a mixture of hydrocarbons. Unsaturated hydrocarbons, which contain one or more double bonds, are useful chemicals for many reactions. | ||
In [[organic chemistry]], hydrocarbons may be classified into two categories, ''[[aromatic hydrocarbons]]'' (see below) that contain one or more [[benzene]] rings or similar rings and ''[[aliphatic hydrocarbon]]s'' which includes all other types of hydrocarbons (see below). | |||
== Linear saturated hydrocarbons == | == Linear saturated hydrocarbons == | ||
| Line 8: | Line 10: | ||
{| class = "wikitable" | {| class = "wikitable" | ||
|+ Some example alkanes | |+ Some example alkanes <ref name=56thHndbk>{{cite book|author=Robert C. Weast (Editor)|title=Handbook of Chemistry and Physics|edition=56th Edition|publisher=CRC Press|year=1975|id=ISBN 0-87819-455-X}}</ref> | ||
! Name!!Formula!!C<sub>n</sub>H<sub>2n+2</sub> | ! Name!!Formula!!C<sub>n</sub>H<sub>2n+2</sub> | ||
|- | |- | ||
| Line 39: | Line 41: | ||
{| class = "wikitable" | {| class = "wikitable" | ||
|+ Some example alkenes | |+ Some example alkenes <ref name=56thHndbk/> | ||
! Name!!Formula!!C<sub>n</sub>H<sub>2n</sub> | ! Name!!Formula!!C<sub>n</sub>H<sub>2n</sub> | ||
|- | |- | ||
| [[Ethene]]|| CH<sub>2</sub>=CH<sub>2</sub>||C<sub>2</sub>H<sub>4</sub> | | [[Ethene]]|| CH<sub>2</sub>=CH<sub>2</sub>||C<sub>2</sub>H<sub>4</sub> | ||
|- | |- | ||
| [[Propene]] || CH<sub>2</sub>= | | [[Propene]] || CH<sub>2</sub>=CH–CH<sub>3</sub>||C<sub>3</sub>H<sub>6</sub> | ||
|- | |- | ||
| [[1-Butene]]|| CH<sub>2</sub>= | | [[1-Butene]]|| CH<sub>2</sub>=CH–CH<sub>2</sub>–CH<sub>3</sub>||C<sub>4</sub>H<sub>8</sub> | ||
|- | |- | ||
| [[2-Butene]]|| CH<sub>3</sub> | | [[2-Butene]]|| CH<sub>3</sub>–CH<sub></sub>=CH<sub></sub>–CH<sub>3</sub> ||C<sub>4</sub>H<sub>8</sub> | ||
|- | |- | ||
| [[1-Pentene]]|| CH<sub>2</sub>= | | [[1-Pentene]]|| CH<sub>2</sub>=CH–(CH<sub>2</sub>)<sub>2</sub>–CH<sub>3</sub>||C<sub>5</sub>H<sub>10</sub> | ||
|- | |- | ||
| [[1-Hexene]]|| CH<sub>2</sub>= | | [[1-Hexene]]|| CH<sub>2</sub>=CH–(CH<sub>2</sub>)<sub>3</sub>–CH<sub>3</sub>||C<sub>6</sub>H<sub>12</sub> | ||
|} | |} | ||
Linear unsaturated hydrocarbons containing two double bonds are referred to as '''dienes''', '''diolefins''' or '''alkadienes'''. Their general formula is C<sub>n</sub>H<sub>2(n-1)</sub> and some example dienes are: | Linear unsaturated hydrocarbons containing two double bonds are referred to as '''dienes''', '''diolefins''' or '''alkadienes'''. Their general formula is C<sub>n</sub>H<sub>2(n-1)</sub> and some example dienes are:<ref name=56thHndbk/> | ||
* [[1,2-Butadiene]]: CH<sub>2</sub>=C= | * [[1,2-Butadiene]]: CH<sub>2</sub>=C=CH–CH<sub>3</sub> or C<sub>4</sub>H<sub>6</sub> | ||
* [[1,2-Pentadiene]]: CH<sub>2</sub>=C= | * [[1,2-Pentadiene]]: CH<sub>2</sub>=C=CH–CH<sub>2</sub>–CH<sub>3</sub> or C<sub>5</sub>H<sub>8</sub> | ||
Linear unsaturated hydrocarbons containing a triple bond are referred to as '''alkynes'''. The simplest example is [[ethyne]] ([[acetylene]]): | Linear unsaturated hydrocarbons containing a triple bond are referred to as '''alkynes'''. The simplest example is [[ethyne]] ([[acetylene]]): H–C≡C–H or C<sub>2</sub>H<sub>2</sub> | ||
== Cyclic saturated hydrocarbons == | == Cyclic saturated hydrocarbons == | ||
{{Image|Example Cycloalkanes.png|right|285px|Example cycloalkanes.<ref name=56thHndbk/>}} | |||
Cyclic saturated hydrocarbons have a closed ring of carbon atoms in a polygon configuration having the same number of vertices as the number of carbon atoms, with each carbon atom being bonded to two hydrogen atoms and to two other carbon atoms. They are referred to as '''cycloalkanes''', '''cycloparaffins''' or '''naphthenes'''. | |||
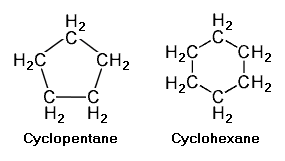
Cycloalkanes with a single ring of carbon atoms have a general formula of C<sub>n</sub>H<sub>2n</sub>. The adjacent image depicts two such single ring cycloalkanes, namely [[cyclopentane]] (C<sub>5</sub>H<sub>10</sub>) and [[cyclohexane]] (C<sub>6</sub>H<sub>12</sub>). | |||
Cycloalkanes may also have two fused (i.e., conjoined) rings. For example, two cyclohexane rings may be fused, so that two of the carbon atoms are shared by each of the two rings, to form [[decalin]] (C<sub>10</sub>H<sub>18</sub>) which is referred to as a '''bicycloalkane'''. | |||
The general formula for cycloalkanes is C<sub>n</sub>H<sub>2(n+1-g)</sub> where g is the number of rings. | |||
== Cyclic unsaturated hydrocarbons == | |||
{{Image|Example Cycloalkenes.png|right|278px|Example cycloalkenes.<ref name=56thHndbk/>}} | |||
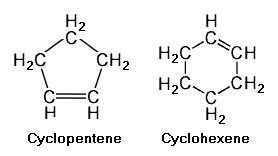
Cyclic | Cyclic unsaturated hydrocarbons have a closed ring of carbon atoms in a polygon configuration having the same number of vertices as the number of carbon atoms. If the hydrocarbons have a ring containing a single double bond between two of the carbons, the hydrocarbons are referred to as '''cycloalkenes''' or '''cycloolefins'''. The adjacent image depicts two such cycloalkenes with single double bonds, namely [[cyclopentene]] (C<sub>5</sub>H<sub>8</sub>) and [[cyclohexene]] (C<sub>6</sub>H<sub>10</sub>). | ||
The general formula for single-ring cycloalkenes with a single double bond is C<sub>n</sub>H<sub>2n-2</sub>. | |||
If single-ring alkenes have two double bonds, they are referred to as '''cyclodienes''' or '''cyclodiolefins'''. An example is [[cyclopentadiene]] (C<sub>5</sub>H<sub>6</sub>), and the general formula for single-ring cycloalkenes with two double bonds is C<sub>n</sub>H<sub>2n-4</sub>. | |||
== Aromatic hydrocarbons == | == Aromatic hydrocarbons == | ||
{{Image|Example Aromatics.png|right|396px|Example aromatics.<ref name=56thHndbk/>}} | |||
{{see also|Aromatic hydrocarbons}} | |||
Aromatic hydrocarbons have a closed ring of 6 carbon atoms in the shape of a hexagon with 3 of the carbon atoms having a double bond with one connected carbon atom and a single bond with another connected carbon. Such a ring is referred to as a ''[[benzene]] ring'' and all hydrocarbons containing one or more such rings are referred to as '''aromatics''' or '''aryl compounds'''. | |||
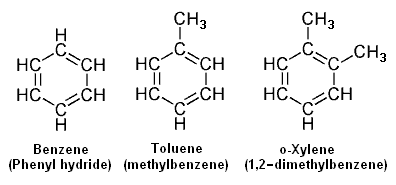
The adjacent image depicts three of the most common aromatics, namely benzene (C<sub>6</sub>H<sub>6</sub>), [[toluene]] (C<sub>7</sub>H<sub>8</sub> and [[o-xylene]] (C<sub>8</sub>H<sub>10</sub>). There are three possible [[xylene]]s referred to as ortho-xylene (o-xylene), meta-xylene ([[m-xylene]]) and para-xylene ([[p-xylene]]). | |||
Aromatics may have two or more rings, either in fused or other configurations, and may have many different side groups or side chains (such as the methyl side group in toluene). There are quite literally hundreds (if not thousands) of various aromatic hydrocarbons. | |||
== References == | |||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 30 August 2024
Hydrocarbons are a class of molecules that contain only carbon and hydrogen atoms. Some of them make very good fuels. Gasoline contains a mixture of hydrocarbons. Unsaturated hydrocarbons, which contain one or more double bonds, are useful chemicals for many reactions.
In organic chemistry, hydrocarbons may be classified into two categories, aromatic hydrocarbons (see below) that contain one or more benzene rings or similar rings and aliphatic hydrocarbons which includes all other types of hydrocarbons (see below).
Linear saturated hydrocarbons
The simplest hydrocarbons are linear molecules in which each carbon atoms is bonded to two other carbons atoms, in a linear fashion, except for the carbon atoms at the ends, which are only bonded to one other carbon atom. Saturated hydrocarbon names generally end with the suffix "ane" which distinguishes them from unsaturated hydrocarbons, which end with the suffix "ene".
Linear saturated hydrocarbons are referred to as paraffins or alkanes. Their general formula is CnHn+2.
| Name | Formula | CnH2n+2 |
|---|---|---|
| Methane | CH4 | CH4 |
| Ethane | CH3–CH3 | C2H6 |
| Propane | CH3–CH2–CH3 | C3H8 |
| Butane | CH3–(CH2)2–CH3 | C4H10 |
| Pentane | CH3–(CH2)3–CH3 | C5H12 |
| Hexane | CH3–(CH2)4–CH3 | C6H14 |
| Heptane | CH3–(CH2)5–CH3 | C7H16 |
| Octane | CH3–(CH2)6–CH3 | C8H18 |
| Nonane | CH3–(CH2)7–CH3 | C9H20 |
| Decane | CH3–(CH2)8–CH3 | C10H22 |
Linear unsaturated hydrocarbons
Unsaturated hydrocarbons are useful precursor molecules for many reactions. Because they contain one or more double bonds, a large variety of chemical transformations are possible. Unsaturated hydrocarbons generally end with the "ene" suffix, although common names are sometimes used instead of the IUPAC designation. In addition, a numerical prefix is used to indicate the position of the double bond(s).
Linear unsaturated hydrocarbons containing a single double bond are referred to as olefins or alkenes. Their general formula is CnH2n.
| Name | Formula | CnH2n |
|---|---|---|
| Ethene | CH2=CH2 | C2H4 |
| Propene | CH2=CH–CH3 | C3H6 |
| 1-Butene | CH2=CH–CH2–CH3 | C4H8 |
| 2-Butene | CH3–CH=CH–CH3 | C4H8 |
| 1-Pentene | CH2=CH–(CH2)2–CH3 | C5H10 |
| 1-Hexene | CH2=CH–(CH2)3–CH3 | C6H12 |
Linear unsaturated hydrocarbons containing two double bonds are referred to as dienes, diolefins or alkadienes. Their general formula is CnH2(n-1) and some example dienes are:[1]
- 1,2-Butadiene: CH2=C=CH–CH3 or C4H6
- 1,2-Pentadiene: CH2=C=CH–CH2–CH3 or C5H8
Linear unsaturated hydrocarbons containing a triple bond are referred to as alkynes. The simplest example is ethyne (acetylene): H–C≡C–H or C2H2
Cyclic saturated hydrocarbons
Cyclic saturated hydrocarbons have a closed ring of carbon atoms in a polygon configuration having the same number of vertices as the number of carbon atoms, with each carbon atom being bonded to two hydrogen atoms and to two other carbon atoms. They are referred to as cycloalkanes, cycloparaffins or naphthenes.
Cycloalkanes with a single ring of carbon atoms have a general formula of CnH2n. The adjacent image depicts two such single ring cycloalkanes, namely cyclopentane (C5H10) and cyclohexane (C6H12).
Cycloalkanes may also have two fused (i.e., conjoined) rings. For example, two cyclohexane rings may be fused, so that two of the carbon atoms are shared by each of the two rings, to form decalin (C10H18) which is referred to as a bicycloalkane.
The general formula for cycloalkanes is CnH2(n+1-g) where g is the number of rings.
Cyclic unsaturated hydrocarbons
Cyclic unsaturated hydrocarbons have a closed ring of carbon atoms in a polygon configuration having the same number of vertices as the number of carbon atoms. If the hydrocarbons have a ring containing a single double bond between two of the carbons, the hydrocarbons are referred to as cycloalkenes or cycloolefins. The adjacent image depicts two such cycloalkenes with single double bonds, namely cyclopentene (C5H8) and cyclohexene (C6H10).
The general formula for single-ring cycloalkenes with a single double bond is CnH2n-2.
If single-ring alkenes have two double bonds, they are referred to as cyclodienes or cyclodiolefins. An example is cyclopentadiene (C5H6), and the general formula for single-ring cycloalkenes with two double bonds is CnH2n-4.
Aromatic hydrocarbons
- See also: Aromatic hydrocarbons
Aromatic hydrocarbons have a closed ring of 6 carbon atoms in the shape of a hexagon with 3 of the carbon atoms having a double bond with one connected carbon atom and a single bond with another connected carbon. Such a ring is referred to as a benzene ring and all hydrocarbons containing one or more such rings are referred to as aromatics or aryl compounds.
The adjacent image depicts three of the most common aromatics, namely benzene (C6H6), toluene (C7H8 and o-xylene (C8H10). There are three possible xylenes referred to as ortho-xylene (o-xylene), meta-xylene (m-xylene) and para-xylene (p-xylene).
Aromatics may have two or more rings, either in fused or other configurations, and may have many different side groups or side chains (such as the methyl side group in toluene). There are quite literally hundreds (if not thousands) of various aromatic hydrocarbons.