Atenolol: Difference between revisions
imported>David E. Volk mNo edit summary |
Pat Palmer (talk | contribs) mNo edit summary |
||
| (29 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Chem infobox | {{Chem infobox | ||
| Line 16: | Line 15: | ||
|casnumber= 29122-68-7 | |casnumber= 29122-68-7 | ||
}} | }} | ||
In [[medicine]], '''atenolol''' is a cardioselective [[adrenergic beta-antagonist]] that is "possessing properties and potency similar to [[propranolol]], but without a negative inotropic effect."<ref>{{MeSH}}</ref> Atenolol is hydrophilic<ref name="pmid8521562">{{cite journal |author=Tuininga YS, Crijns HJ, Brouwer J, ''et al'' |title=Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients |journal=Circulation |volume=92 |issue=12 |pages=3415–23 |year=1995 |month=December |pmid=8521562 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=8521562 |issn=}}</ref> | In [[medicine]], '''atenolol''' is a cardioselective [[adrenergic beta-antagonist]] that is "possessing properties and potency similar to [[propranolol]], but without a negative inotropic effect."<ref>{{MeSH}}</ref> Atenolol is hydrophilic<ref name="pmid8521562">{{cite journal |author=Tuininga YS, Crijns HJ, Brouwer J, ''et al'' |title=Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients |journal=Circulation |volume=92 |issue=12 |pages=3415–23 |year=1995 |month=December |pmid=8521562 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=8521562 |issn=}}</ref> | ||
==History== | ==History== | ||
Atenolol was developed by the [http://www.astrazenecacareers.com/content/aboutAZ/ourCompany/ourHistory/astrazeneca-our-history-corporate-evolution.asp#stuart Stuart Company] which was a division of Imperial Chemical Industries (ICI). ICI was renamed Zeneca in 1992. Atenolol received approval in the [[United States]] August 19, 1981.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search. | Atenolol was developed by the [http://www.astrazenecacareers.com/content/aboutAZ/ourCompany/ourHistory/astrazeneca-our-history-corporate-evolution.asp#stuart Stuart Company] which was a division of Imperial Chemical Industries (ICI). ICI was renamed Zeneca in 1992. Atenolol received approval in the [[United States of America]] August 19, 1981.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.SearchAction&SearchType=BasicSearch&Search_Button=Submit&searchTerm=018240 Drugs@FDA]. U S Food and Drug Administration</ref> According to drugstore.com, 90 days of generic 50 mg pills costs $17.99 in January, 2009. | ||
[[Generic drug|Generic]] atenolol was available in 1988.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.SearchAction&SearchType=BasicSearch&Search_Button=Submit&searchTerm=072303 Drugs@FDA]. U S Food and Drug Administration</ref> | |||
== Chemistry == | |||
Atenolol may defined by [[IUPAC]] nomenclature: | |||
* 4-[2'-hydroxy-3'-[(1-methylethyl)amino]propoxy-benzeneacetamide | |||
* Benzeneacetamide, 4-[2'-hydroxy-3'-[(1-methylethyl) amino] propoxy]- (according to [http://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=AtenololDailyMed]) | |||
It is a [[hydrophilic]] drug, with solubility in water equal to 26.5 mg/mL at 37ºC, with chemical formula C<sub>14</sub>H<sub>22</sub>N<sub>2</sub>O<sub>3</sub> and[[molecular mass]] 266.34 gram/mole for the free base form. It is freely soluble in strongly acidic solutions. | |||
==Metabolism== | ==Metabolism== | ||
Atenolol is excreted unchanged in the kidneys. Elimination is dependent on the [[glomerular filtration rate]]. Atenolol is ''not'' metabolized in the liver by [[cytochrome P-450]] [http://www.ncbi.nlm.nih.gov/sites/entrez/?db=gene&cmd=Retrieve&dopt=summary&list_uids=1565 2D6] [[allele]]. | Atenolol is excreted unchanged in the kidneys. Elimination is dependent on the [[glomerular filtration rate]]. Atenolol is ''not'' metabolized in the liver by [[cytochrome P-450]] [http://www.ncbi.nlm.nih.gov/sites/entrez/?db=gene&cmd=Retrieve&dopt=summary&list_uids=1565 2D6] [[allele]]. | ||
With normal renal function, the serum [[half-life]] is about 8 hours. While it was originally thought<ref name="isbn0-683-04522-9">{{cite book |author=Kaplan, Norman M.; Lieberman, Ellin |authorlink= |editor= |others= |title=Clinical Hypertension|chapter=Treatment of Hypertension: Drug Therapy |edition=5th|language= |publisher=Williams & Wilkins |location=Baltimore |year=1990 |origyear= |pages=220 |quote=All beta-blockers act longer on the blood pressure than the pharmacokinetic data would imply. In moderate doses, most beta-blockers will likely keep the blood pressure down when given once daily. To ensure adequate control, early morning blood pressures should be measured before the daily dose is taken |isbn=0-683-04522-9 |oclc= |doi= |url= |accessdate=}}</ref> and promoted<ref name="isbn1-56363-703-0">{{cite book |author=Physicians Desk Reference |authorlink= |editor= |others= |title=Physicians' Desk Reference |edition=36th |language=English |publisher=Medical Economics |location=Oradell, NJ |year=1982|origyear= |pages=1884 |quote= |isbn=0874898501 |oclc= |doi= |url= |accessdate=}}</ref> that atenolol can be used once a day for isolated hypertension because the [[central nervous system]] pharmacodynamic effect persists longer, subsequent studies suggest atenolol should be dosed twice a day even for hypertension.<ref name="isbn0-683-04544-X">{{cite book |author=Kaplan, Norman M.; Lieberman, Ellin |authorlink= |editor= |others= |title=Clinical Hypertension |edition=6th|chapter=Treatment of Hypertension: Drug Therapy |language= |publisher=Williams & Wilkins |location=Baltimore |year=1994 |origyear= |pages=225 |quote=In the usual doses prescribed, various beta blockers have equal antihypertensive efficacy. However, they many not all provide full 24-hour lowering of the BP which may be particularly critical in protecting against early morning cardiovascular catastrophes. Metoprolol blunted this rise, but atenolol and pindolol did not (Raftery and Carrageta, 1985). Neutel et al, (1990) found a similar lack of 24-hour effect with once-daily atenolol but a sustained effect with acebutolol. Moreover, twice-daily doses of "cardioselective" agents may preserve this cardioselectivity better than once-daily large doses (Lipworth et al, 1991).|isbn=0-683-04544-X |oclc= |doi= |url= |accessdate=}}</ref><ref name="pmid18259123">{{cite journal |author=Sarafidis P, Bogojevic Z, Basta E, Kirstner E, Bakris GL |title=Comparative efficacy of two different beta-blockers on 24-hour blood pressure control |journal=J Clin Hypertens (Greenwich) |volume=10 |issue=2 |pages=112–8 |year=2008 |month=February |pmid=18259123 |doi=10.1111/j.1751-7176.2008.08021.x |url= |issn=}}</ref> | |||
==Dosage== | ==Dosage== | ||
For healthy adults, the starting dose recommended by the manufacturer is 50 mg orally once daily | For healthy adults, the starting dose recommended by the manufacturer is 50 mg orally once daily<ref name="isbn1-56363-703-0">{{cite book |author=Physicians Desk Reference |authorlink= |editor= |others= |title=Physicians' Desk Reference |edition=36th |language=English |publisher=Medical Economics |location=Oradell, NJ |year=1982|origyear= |pages=1884 |quote= |isbn=0874898501 |oclc= |doi= |url= |accessdate=}}</ref> and the maximum dose is 100 mg orally once daily. If the estimated creatinine clearance is less than 15 ml/min then the dose should be given every other day<ref name="isbn1-56363-703-0"/> or the dose should be 25 mg once per day<ref>[http://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Atenolol Atenolol] - FDA approved drug information (drug label) from [http://dailymed.nlm.nih.gov/dailymed/ DailyMed] (U.S. [[National Library of Medicine]]). </ref>. | ||
== | ''However'', atenolol may require twice daily dosing<ref name="pmid2360502">{{cite journal |author=Neutel JM, Schnaper H, Cheung DG, Graettinger WF, Weber MA |title=Antihypertensive effects of beta-blockers administered once daily: 24-hour measurements |journal=Am. Heart J. |volume=120 |issue=1 |pages=166–71 |year=1990 |month=July |pmid=2360502 |doi=10.1016/0002-8703(90)90174-V |url=http://linkinghub.elsevier.com/retrieve/pii/0002-8703(90)90174-V |issn=}}</ref><ref name="pmid18259123">{{cite journal |author=Sarafidis P, Bogojevic Z, Basta E, Kirstner E, Bakris GL |title=Comparative efficacy of two different beta-blockers on 24-hour blood pressure control |journal=J Clin Hypertens (Greenwich) |volume=10 |issue=2 |pages=112–8 |year=2008 |month=February |pmid=18259123 |doi=10.1111/j.1751-7176.2008.08021.x |url= |issn=}}</ref> The INVEST trial used atenolol twice a day if 50 mg once per day did not control pressure. In this study atenolol had similar outcomes to other [[Antihypertensive|antihypertensive agent]]s.<ref name="pmid14657064">{{cite journal |author=Pepine CJ, Handberg EM, Cooper-DeHoff RM, ''et al.'' |title=A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial |journal=JAMA |volume=290 |issue=21 |pages=2805–16 |year=2003 |month=December |pmid=14657064 |doi=10.1001/jama.290.21.2805 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=14657064 |issn=}}</ref> | ||
==Efficacy== | ==Efficacy== | ||
=== | ===Acute myocardial infarction=== | ||
{{Image|Atenolol for acute myocardial infarction - meta-analysis.jpg|right|350px|Atenolol for acute [[myocardial infarction]] - [[meta-analysis]] of atenolol subgroup from larger meta-analysis by Brandler.<ref name="pmid20078433">{{cite journal| author=Brandler E, Paladino L, Sinert R| title=Does the early administration of beta-blockers improve the in-hospital mortality rate of patients admitted with acute coronary syndrome? | journal=Acad Emerg Med | year= 2010 | volume= 17 | issue= 1 | pages= 1-10 | pmid=20078433 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=20078433 | doi=10.1111/j.1553-2712.2009.00625.x }} </ref>.}} | |||
Although atenolol is thought to be less effective than other beta-blockers for acute treatment according to [[systematic review]]s<ref name="pmid10381708">{{cite journal |author=Freemantle N, Cleland J, Young P, Mason J, Harrison J |title=beta Blockade after myocardial infarction: systematic review and meta regression analysis |journal=BMJ |volume=318 |issue=7200 |pages=1730–7 |year=1999 |month=June |pmid=10381708 |pmc=31101 |doi= |url=http://bmj.com/cgi/pmidlookup?view=long&pmid=10381708 |issn=}}</ref> of three randomized controlled trials. These reviews are dominated by the ISIS-1 study which found that atenolol reduced mortality, though not significantly so<ref name="pmid2873379">{{cite journal |author= |title=Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group |journal=Lancet |volume=2 |issue=8498 |pages=57–66 |year=1986 |month=July |pmid=2873379 |doi= |url= |issn=}}</ref>. However, atenolol was dosed at 100 mg per day and the Freemantel and co-meta-analysts studied the outcomes of the ISIS-1 at on year although the intervention lasted 7 days.<ref name="pmid2873379"/> The other two trials dose atenolol at twice a day.<ref name="pmid6851037">{{cite journal| author=Yusuf S, Sleight P, Rossi P, Ramsdale D, Peto R, Furze L et al.| title=Reduction in infarct size, arrhythmias and chest pain by early intravenous beta blockade in suspected acute myocardial infarction. | journal=Circulation | year= 1983 | volume= 67 | issue= 6 Pt 2 | pages= I32-41 | pmid=6851037 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=6851037 }}</ref><ref name="pmid8335810">{{cite journal| author=Van de Werf F, Janssens L, Brzostek T, Mortelmans L, Wackers FJ, Willems GM et al.| title=Short-term effects of early intravenous treatment with a beta-adrenergic blocking agent or a specific bradycardiac agent in patients with acute myocardial infarction receiving thrombolytic therapy. | journal=J Am Coll Cardiol | year= 1993 | volume= 22 | issue= 2 | pages= 407-16 | pmid=8335810 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=8335810 }}</ref> | |||
[[Cohort study|Cohort studies]] suggest that atenolol may<ref name="pmid17239680">{{cite journal| author=Rinfret S, Abrahamowicz M, Tu J, Humphries K, Eisenberg MJ, Richard H et al.| title=A population-based analysis of the class effect of beta-blockers after myocardial infarction. | journal=Am Heart J | year= 2007 | volume= 153 | issue= 2 | pages= 224-30 | pmid=17239680 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=17239680 | doi=10.1016/j.ahj.2006.11.008 }} </ref> or may not<ref name="pmid18612201">{{cite journal| author=Andersen SS, Hansen ML, Gislason GH, Folke F, Schramm TK, Fosbøl E et al.| title=Mortality and reinfarction among patients using different beta-blockers for secondary prevention after a myocardial infarction. | journal=Cardiology | year= 2009 | volume= 112 | issue= 2 | pages= 144-50 | pmid=18612201 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=18612201 | doi=10.1159/000143389 }} </ref> be better than other [[adrenergic beta-antagonist]]s. | |||
===Heart failure=== | ===Heart failure=== | ||
Although atenolol has not received indication in the United States for the treatment of heart failure, two [[cohort study|cohort studies]] suggest that the [[beta-blocker]]s [[atenolol]] and [[carvedilol]] may be more effect than [[metoprolol]] for the treatment of heart failure.<ref name="pmid19064824">{{cite journal |author=Kramer JM, Curtis LH, Dupree CS, ''et al'' |title=Comparative effectiveness of beta-blockers in elderly patients with heart failure |journal=Arch. Intern. Med. |volume=168 |issue=22 |pages=2422–8; discussion 2428–32 |year=2008 |month=December |pmid=19064824 |doi=10.1001/archinternmed.2008.511 |url=http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=19064824 |issn=}}</ref><ref name="pmid19064823">{{cite journal |author=Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R |title=Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice |journal=Arch. Intern. Med. |volume=168 |issue=22 |pages=2415–21 |year=2008 |month=December |pmid=19064823 |doi=10.1001/archinternmed.2008.506 |url=http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=19064823 |issn=}}</ref> | Although atenolol has not received indication in the United States for the treatment of [[heart failure]], two [[cohort study|cohort studies]] suggest that the [[beta-blocker]]s [[atenolol]] and [[carvedilol]] may be more effect than [[metoprolol]] for the treatment of heart failure.<ref name="pmid19064824">{{cite journal |author=Kramer JM, Curtis LH, Dupree CS, ''et al'' |title=Comparative effectiveness of beta-blockers in elderly patients with heart failure |journal=Arch. Intern. Med. |volume=168 |issue=22 |pages=2422–8; discussion 2428–32 |year=2008 |month=December |pmid=19064824 |doi=10.1001/archinternmed.2008.511 |url=http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=19064824 |issn=}}</ref><ref name="pmid19064823">{{cite journal |author=Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R |title=Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice |journal=Arch. Intern. Med. |volume=168 |issue=22 |pages=2415–21 |year=2008 |month=December |pmid=19064823 |doi=10.1001/archinternmed.2008.506 |url=http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=19064823 |issn=}}</ref> | ||
[[Randomized controlled trial]]s by one research group also suggest atenolol might benefit.<ref name="pmid11113718">{{cite journal |author=Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B |title=Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril |journal=Eur. J. Heart Fail. |volume=2 |issue=4 |pages=407–12 |year=2000 |month=December |pmid=11113718 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S1388984200001203 |issn=}}</ref><ref name="pmid11704477">{{cite journal |author=Hülsmann M, Sturm B, Pacher R, ''et al'' |title=Long-term effect of atenolol on ejection fraction, symptoms, and exercise variables in patients with advanced left ventricular dysfunction |journal=J. Heart Lung Transplant. |volume=20 |issue=11 |pages=1174–80 |year=2001 |month=November |pmid=11704477 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S1053-2498(01)00341-2 |issn=}}</ref> | [[Randomized controlled trial]]s by one research group also suggest atenolol might benefit.<ref name="pmid11113718">{{cite journal |author=Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B |title=Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril |journal=Eur. J. Heart Fail. |volume=2 |issue=4 |pages=407–12 |year=2000 |month=December |pmid=11113718 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S1388984200001203 |issn=}}</ref><ref name="pmid11704477">{{cite journal |author=Hülsmann M, Sturm B, Pacher R, ''et al'' |title=Long-term effect of atenolol on ejection fraction, symptoms, and exercise variables in patients with advanced left ventricular dysfunction |journal=J. Heart Lung Transplant. |volume=20 |issue=11 |pages=1174–80 |year=2001 |month=November |pmid=11704477 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S1053-2498(01)00341-2 |issn=}}</ref> | ||
===Hypertension=== | ===Hypertension=== | ||
Atenolol is commonly used to treat [[hypertension]]. However, it may not reduce mortality as well as other antihypertensives according to a systematic review; however, this review did not compare atenolol against other [[adrenergic beta-antagonist]]s and it is not clear whether all of the included trials allowed patients with active coronary heart disease who would have most benefitted from a [[adrenergic beta-antagonist]].<ref name="pmid15530629">{{cite journal |author=Carlberg B, Samuelsson O, Lindholm LH |title=Atenolol in hypertension: is it a wise choice? |journal=Lancet |volume=364 |issue=9446 |pages=1684–9 |year=2004 |pmid=15530629 |doi=10.1016/S0140-6736(04)17355-8 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(04)17355-8 |issn=}}</ref> | |||
The INVEST trial used atenolol ''twice'' a day if 50 mg once per day did not control pressure.<ref name="pmid14657064">{{cite journal |author=Pepine CJ, Handberg EM, Cooper-DeHoff RM, ''et al.'' |title=A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial |journal=JAMA |volume=290 |issue=21 |pages=2805–16 |year=2003 |month=December |pmid=14657064 |doi=10.1001/jama.290.21.2805 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=14657064 |issn=}}</ref> In this study atenolol had similar outcomes to other antihypertensive agents; however patients were included if they had [[coronary heart disease]] and benefit was confined to patients with prior [[myocardial infarction]].<ref name="pmid18375982">{{cite journal |author=Kolloch R, Legler UF, Champion A, ''et al.'' |title=Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST) |journal=Eur. Heart J. |volume=29 |issue=10 |pages=1327–34 |year=2008 |month=May |pmid=18375982 |doi=10.1093/eurheartj/ehn123 |url=http://eurheartj.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=18375982 |issn=}}</ref> | |||
==External links== | ==External links== | ||
| Line 45: | Line 66: | ||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 13:01, 27 July 2024
|
| |||||||
| atenolol | |||||||
| |||||||
| Uses: | hypertention;angina pectoris | ||||||
| Properties: | hydrophilic | ||||||
| Hazards: | see side effects & drug interactions | ||||||
| |||||||
In medicine, atenolol is a cardioselective adrenergic beta-antagonist that is "possessing properties and potency similar to propranolol, but without a negative inotropic effect."[1] Atenolol is hydrophilic[2]
History
Atenolol was developed by the Stuart Company which was a division of Imperial Chemical Industries (ICI). ICI was renamed Zeneca in 1992. Atenolol received approval in the United States of America August 19, 1981.[3] According to drugstore.com, 90 days of generic 50 mg pills costs $17.99 in January, 2009.
Generic atenolol was available in 1988.[4]
Chemistry
Atenolol may defined by IUPAC nomenclature:
- 4-[2'-hydroxy-3'-[(1-methylethyl)amino]propoxy-benzeneacetamide
- Benzeneacetamide, 4-[2'-hydroxy-3'-[(1-methylethyl) amino] propoxy]- (according to [1])
It is a hydrophilic drug, with solubility in water equal to 26.5 mg/mL at 37ºC, with chemical formula C14H22N2O3 andmolecular mass 266.34 gram/mole for the free base form. It is freely soluble in strongly acidic solutions.
Metabolism
Atenolol is excreted unchanged in the kidneys. Elimination is dependent on the glomerular filtration rate. Atenolol is not metabolized in the liver by cytochrome P-450 2D6 allele.
With normal renal function, the serum half-life is about 8 hours. While it was originally thought[5] and promoted[6] that atenolol can be used once a day for isolated hypertension because the central nervous system pharmacodynamic effect persists longer, subsequent studies suggest atenolol should be dosed twice a day even for hypertension.[7][8]
Dosage
For healthy adults, the starting dose recommended by the manufacturer is 50 mg orally once daily[6] and the maximum dose is 100 mg orally once daily. If the estimated creatinine clearance is less than 15 ml/min then the dose should be given every other day[6] or the dose should be 25 mg once per day[9].
However, atenolol may require twice daily dosing[10][8] The INVEST trial used atenolol twice a day if 50 mg once per day did not control pressure. In this study atenolol had similar outcomes to other antihypertensive agents.[11]
Efficacy
Acute myocardial infarction
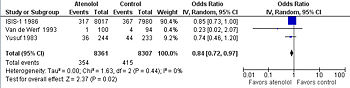
Although atenolol is thought to be less effective than other beta-blockers for acute treatment according to systematic reviews[13] of three randomized controlled trials. These reviews are dominated by the ISIS-1 study which found that atenolol reduced mortality, though not significantly so[14]. However, atenolol was dosed at 100 mg per day and the Freemantel and co-meta-analysts studied the outcomes of the ISIS-1 at on year although the intervention lasted 7 days.[14] The other two trials dose atenolol at twice a day.[15][16]
Cohort studies suggest that atenolol may[17] or may not[18] be better than other adrenergic beta-antagonists.
Heart failure
Although atenolol has not received indication in the United States for the treatment of heart failure, two cohort studies suggest that the beta-blockers atenolol and carvedilol may be more effect than metoprolol for the treatment of heart failure.[19][20]
Randomized controlled trials by one research group also suggest atenolol might benefit.[21][22]
Hypertension
Atenolol is commonly used to treat hypertension. However, it may not reduce mortality as well as other antihypertensives according to a systematic review; however, this review did not compare atenolol against other adrenergic beta-antagonists and it is not clear whether all of the included trials allowed patients with active coronary heart disease who would have most benefitted from a adrenergic beta-antagonist.[23]
The INVEST trial used atenolol twice a day if 50 mg once per day did not control pressure.[11] In this study atenolol had similar outcomes to other antihypertensive agents; however patients were included if they had coronary heart disease and benefit was confined to patients with prior myocardial infarction.[24]
External links
The most up-to-date information about Atenolol and other drugs can be found at the following sites.
- Atenolol - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Atenolol - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Atenolol - Detailed information from DrugBank.
References
- ↑ Anonymous (2024), Atenolol (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Tuininga YS, Crijns HJ, Brouwer J, et al (December 1995). "Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients". Circulation 92 (12): 3415–23. PMID 8521562. [e]
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ Kaplan, Norman M.; Lieberman, Ellin (1990). “Treatment of Hypertension: Drug Therapy”, Clinical Hypertension, 5th. Baltimore: Williams & Wilkins, 220. ISBN 0-683-04522-9. “All beta-blockers act longer on the blood pressure than the pharmacokinetic data would imply. In moderate doses, most beta-blockers will likely keep the blood pressure down when given once daily. To ensure adequate control, early morning blood pressures should be measured before the daily dose is taken”
- ↑ 6.0 6.1 6.2 Physicians Desk Reference (1982). Physicians' Desk Reference (in English), 36th. Oradell, NJ: Medical Economics, 1884. ISBN 0874898501.
- ↑ Kaplan, Norman M.; Lieberman, Ellin (1994). “Treatment of Hypertension: Drug Therapy”, Clinical Hypertension, 6th. Baltimore: Williams & Wilkins, 225. ISBN 0-683-04544-X. “In the usual doses prescribed, various beta blockers have equal antihypertensive efficacy. However, they many not all provide full 24-hour lowering of the BP which may be particularly critical in protecting against early morning cardiovascular catastrophes. Metoprolol blunted this rise, but atenolol and pindolol did not (Raftery and Carrageta, 1985). Neutel et al, (1990) found a similar lack of 24-hour effect with once-daily atenolol but a sustained effect with acebutolol. Moreover, twice-daily doses of "cardioselective" agents may preserve this cardioselectivity better than once-daily large doses (Lipworth et al, 1991).”
- ↑ 8.0 8.1 Sarafidis P, Bogojevic Z, Basta E, Kirstner E, Bakris GL (February 2008). "Comparative efficacy of two different beta-blockers on 24-hour blood pressure control". J Clin Hypertens (Greenwich) 10 (2): 112–8. DOI:10.1111/j.1751-7176.2008.08021.x. PMID 18259123. Research Blogging.
- ↑ Atenolol - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- ↑ Neutel JM, Schnaper H, Cheung DG, Graettinger WF, Weber MA (July 1990). "Antihypertensive effects of beta-blockers administered once daily: 24-hour measurements". Am. Heart J. 120 (1): 166–71. DOI:10.1016/0002-8703(90)90174-V. PMID 2360502. Research Blogging.
- ↑ 11.0 11.1 Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. (December 2003). "A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial". JAMA 290 (21): 2805–16. DOI:10.1001/jama.290.21.2805. PMID 14657064. Research Blogging.
- ↑ Brandler E, Paladino L, Sinert R (2010). "Does the early administration of beta-blockers improve the in-hospital mortality rate of patients admitted with acute coronary syndrome?". Acad Emerg Med 17 (1): 1-10. DOI:10.1111/j.1553-2712.2009.00625.x. PMID 20078433. Research Blogging.
- ↑ Freemantle N, Cleland J, Young P, Mason J, Harrison J (June 1999). "beta Blockade after myocardial infarction: systematic review and meta regression analysis". BMJ 318 (7200): 1730–7. PMID 10381708. PMC 31101. [e]
- ↑ 14.0 14.1 (July 1986) "Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group". Lancet 2 (8498): 57–66. PMID 2873379. [e]
- ↑ Yusuf S, Sleight P, Rossi P, Ramsdale D, Peto R, Furze L et al. (1983). "Reduction in infarct size, arrhythmias and chest pain by early intravenous beta blockade in suspected acute myocardial infarction.". Circulation 67 (6 Pt 2): I32-41. PMID 6851037.
- ↑ Van de Werf F, Janssens L, Brzostek T, Mortelmans L, Wackers FJ, Willems GM et al. (1993). "Short-term effects of early intravenous treatment with a beta-adrenergic blocking agent or a specific bradycardiac agent in patients with acute myocardial infarction receiving thrombolytic therapy.". J Am Coll Cardiol 22 (2): 407-16. PMID 8335810.
- ↑ Rinfret S, Abrahamowicz M, Tu J, Humphries K, Eisenberg MJ, Richard H et al. (2007). "A population-based analysis of the class effect of beta-blockers after myocardial infarction.". Am Heart J 153 (2): 224-30. DOI:10.1016/j.ahj.2006.11.008. PMID 17239680. Research Blogging.
- ↑ Andersen SS, Hansen ML, Gislason GH, Folke F, Schramm TK, Fosbøl E et al. (2009). "Mortality and reinfarction among patients using different beta-blockers for secondary prevention after a myocardial infarction.". Cardiology 112 (2): 144-50. DOI:10.1159/000143389. PMID 18612201. Research Blogging.
- ↑ Kramer JM, Curtis LH, Dupree CS, et al (December 2008). "Comparative effectiveness of beta-blockers in elderly patients with heart failure". Arch. Intern. Med. 168 (22): 2422–8; discussion 2428–32. DOI:10.1001/archinternmed.2008.511. PMID 19064824. Research Blogging.
- ↑ Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R (December 2008). "Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice". Arch. Intern. Med. 168 (22): 2415–21. DOI:10.1001/archinternmed.2008.506. PMID 19064823. Research Blogging.
- ↑ Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B (December 2000). "Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril". Eur. J. Heart Fail. 2 (4): 407–12. PMID 11113718. [e]
- ↑ Hülsmann M, Sturm B, Pacher R, et al (November 2001). "Long-term effect of atenolol on ejection fraction, symptoms, and exercise variables in patients with advanced left ventricular dysfunction". J. Heart Lung Transplant. 20 (11): 1174–80. PMID 11704477. [e]
- ↑ Carlberg B, Samuelsson O, Lindholm LH (2004). "Atenolol in hypertension: is it a wise choice?". Lancet 364 (9446): 1684–9. DOI:10.1016/S0140-6736(04)17355-8. PMID 15530629. Research Blogging.
- ↑ Kolloch R, Legler UF, Champion A, et al. (May 2008). "Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST)". Eur. Heart J. 29 (10): 1327–34. DOI:10.1093/eurheartj/ehn123. PMID 18375982. Research Blogging.