Technetium: Difference between revisions
imported>Anthony.Sebastian (elaborate on 'lightest') |
mNo edit summary |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 17: | Line 17: | ||
'''Technetium''' is a [[Chemical elements|chemical element]], having the [[chemical symbol]] Tc. Its [[atomic number]] (the number of [[proton]]s) is 43. It has a [[Atomic mass#Standard atomic weights of the elements|standard atomic weight]] of 98.9062 g•mol<sup> −1</sup> and is a [[solid]] in its elemental form. | '''Technetium''' is a [[Chemical elements|chemical element]], having the [[chemical symbol]] Tc. Its [[atomic number]] (the number of [[proton]]s) is 43. It has a [[Atomic mass#Standard atomic weights of the elements|standard atomic weight]] of 98.9062 g•mol<sup> −1</sup> and is a [[solid]] in its elemental form. | ||
Technetium is considered to be a member of the "Transition metal" class of elements.<ref>'''Note:''' | Technetium is considered to be a member of the "Transition metal" class of elements.<ref>'''Note:''' Technetium is also sometimes referred to being a member of a ''Synthetic'' or ''Quasi-synthetic'' class of elements.</ref> At a [[pressure]] of 101.325 k[[Pascal (unit)|Pa]] (= 1.0 [[Atmosphere (unit)|atm]]), it has a [[boiling point]] of 4,265 °[[Celsius (unit)|C]] and a [[melting point]] of 2,157 °C. | ||
All the isotopes of technetium are radioactive; 98 is the atomic mass of technetium's longest-lived isotope, <sup>98</sup>Tc (4.12x10<sup>6</sup> years).<ref>[http://periodictable.com/Isotopes/043.98/index.html Technetium Isotope data].</ref> Technetium has the lowest atomic number of the chemical elements that lack a stable isotope. | All the isotopes of technetium are radioactive; 98 is the atomic mass of technetium's longest-lived isotope, <sup>98</sup>Tc (4.12x10<sup>6</sup> years).<ref>[http://periodictable.com/Isotopes/043.98/index.html Technetium Isotope data].</ref> Technetium has the lowest atomic number of the chemical elements that lack a stable isotope. | ||
| Line 23: | Line 23: | ||
Only very small amounts of technetium are found in nature.<ref name=schwochau>Schwochau K. (2000) ''Technetium: chemistry and radiopharmaceutical applications''. Wiley-VCH. ISBN 9783527294961. | [http://books.google.com/books?id=BHjxH8q9iukC&dq=technetium&source=gbs_navlinks_s Google Books preview]. | Only very small amounts of technetium are found in nature.<ref name=schwochau>Schwochau K. (2000) ''Technetium: chemistry and radiopharmaceutical applications''. Wiley-VCH. ISBN 9783527294961. | [http://books.google.com/books?id=BHjxH8q9iukC&dq=technetium&source=gbs_navlinks_s Google Books preview]. | ||
*<font face="Gill Sans MT">See section 3.2 for history of discovery of technetium in Earth's crust.</font></ref> Practically all technetium is produced synthetically as a by-product of the fission of [[Uranium|uranium-235]] in nuclear reactors and it is extracted from the spent reactor fuel rods.<ref>{{cite book|author=John Elmsley|title=Nature's Building Blocks: An A-Z Guide to the Elements|edition=1st Edition|publisher=Oxford University Press|year=2001|id=ISBN 0-19-850341-5}}</ref> | *<font face="Gill Sans MT">See section 3.2 for history of discovery of technetium in Earth's crust.</font></ref> Practically all technetium is produced synthetically as a by-product of the fission of [[Uranium|uranium-235]] in nuclear reactors and it is extracted from the spent reactor fuel rods.<ref>{{cite book|author=John Elmsley|title=Nature's Building Blocks: An A-Z Guide to the Elements|edition=1st Edition|publisher=Oxford University Press|year=2001|id=ISBN 0-19-850341-5}}</ref> | ||
Technetium has many important medical applications: | |||
<blockquote> | |||
<p style="margin-left: 2%; margin-right: 6%; font-size: 1.0em; font-family: Gill Sans MT, Trebuchet MS;">Or consider the element technetium, which has a far lower atomic number of 43 but which was first discovered in Palermo, Sicily in 1937 after being artificially created in a cyclotron machine in Berkeley, California. Over the subsequent years technetium has found its way into every major hospital in the world and is used in a plethora of medical scanning procedures as well as for treating various medical conditions. It was later found that technetium occurs naturally on earth but in absolutely minute amounts. This happens because technetium is a bi-product of the natural decay of uranium and also because it is a bi-product in the operation of nuclear reactors. The second of these sources provides macroscopic amounts of technetium, which allow scientists to study the chemistry of the element in great detail and to make many new and medically useful compounds. There have been entire conferences devoted to the chemistry and uses of technetium.<ref name=scerrinatblog>Scerri E. (2011) [http://blogs.nature.com/soapboxscience/2011/12/07/the-periodic-table-matter-matters The periodic table: matter matters].</ref></p> | |||
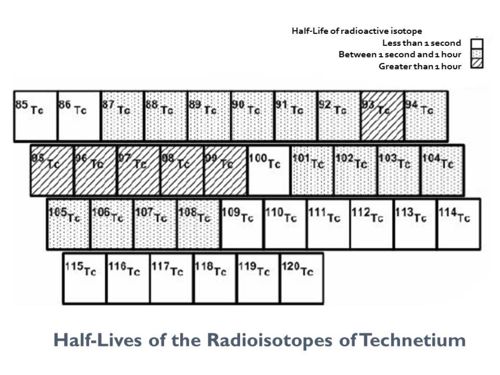
==Technetium radioisotope half-lives== | ==Technetium radioisotope half-lives== | ||
{{Image|Technetium isotopes-3.jpg|center|500px|}} | {{Image|Technetium isotopes-3.jpg|center|500px|}} | ||
{{-}} | {{-}} | ||
To find the numerical value of the half-life of any isotope of technetium, see:<ref>[http://periodictable.com/Elements/043/data.html Technical data for Technetium]. | |||
*<font face="Gill Sans MT">Scroll down to "Nuclear Properties", find "Known Isotopes", click on symbol for any isotope to see its half-life as well other technical data for that isotope, including decay chains leading to and from the isotope.</font></ref> | |||
<ref name=isotcjlab>[http://education.jlab.org/itselemental/iso043.html Isotopes of the Element Technetium]. Thomas Jefferson National Accelerator Laboratory. | Gives mass number, half-life, decay mode, and branching percentage. | See also: [http://www.nndc.bnl.gov/ National Nuclear Data Center].</ref> | |||
==References== | ==References== | ||
{{reflist}} | |||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 25 October 2024
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Technetium is a chemical element, having the chemical symbol Tc. Its atomic number (the number of protons) is 43. It has a standard atomic weight of 98.9062 g•mol −1 and is a solid in its elemental form.
Technetium is considered to be a member of the "Transition metal" class of elements.[1] At a pressure of 101.325 kPa (= 1.0 atm), it has a boiling point of 4,265 °C and a melting point of 2,157 °C.
All the isotopes of technetium are radioactive; 98 is the atomic mass of technetium's longest-lived isotope, 98Tc (4.12x106 years).[2] Technetium has the lowest atomic number of the chemical elements that lack a stable isotope.
Only very small amounts of technetium are found in nature.[3] Practically all technetium is produced synthetically as a by-product of the fission of uranium-235 in nuclear reactors and it is extracted from the spent reactor fuel rods.[4]
Technetium has many important medical applications:
Or consider the element technetium, which has a far lower atomic number of 43 but which was first discovered in Palermo, Sicily in 1937 after being artificially created in a cyclotron machine in Berkeley, California. Over the subsequent years technetium has found its way into every major hospital in the world and is used in a plethora of medical scanning procedures as well as for treating various medical conditions. It was later found that technetium occurs naturally on earth but in absolutely minute amounts. This happens because technetium is a bi-product of the natural decay of uranium and also because it is a bi-product in the operation of nuclear reactors. The second of these sources provides macroscopic amounts of technetium, which allow scientists to study the chemistry of the element in great detail and to make many new and medically useful compounds. There have been entire conferences devoted to the chemistry and uses of technetium.[5]
Technetium radioisotope half-lives
To find the numerical value of the half-life of any isotope of technetium, see:[6] [7]References
- ↑ Note: Technetium is also sometimes referred to being a member of a Synthetic or Quasi-synthetic class of elements.
- ↑ Technetium Isotope data.
- ↑ Schwochau K. (2000) Technetium: chemistry and radiopharmaceutical applications. Wiley-VCH. ISBN 9783527294961. | Google Books preview.
- See section 3.2 for history of discovery of technetium in Earth's crust.
- ↑ John Elmsley (2001). Nature's Building Blocks: An A-Z Guide to the Elements, 1st Edition. Oxford University Press. ISBN 0-19-850341-5.
- ↑ Scerri E. (2011) The periodic table: matter matters.
- ↑ Technical data for Technetium.
- Scroll down to "Nuclear Properties", find "Known Isotopes", click on symbol for any isotope to see its half-life as well other technical data for that isotope, including decay chains leading to and from the isotope.
- ↑ Isotopes of the Element Technetium. Thomas Jefferson National Accelerator Laboratory. | Gives mass number, half-life, decay mode, and branching percentage. | See also: National Nuclear Data Center.