Flue gas emissions from fossil fuel combustion: Difference between revisions
imported>Milton Beychok m (Creating a new article in progress) |
mNo edit summary |
||
| (23 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{subpages}} | {{subpages}} | ||
[[Image:Four Corners Power Plant.jpg|right|thumb|200px|{{#ifexist:Template:Four Corners Power Plant.jpg/credit|{{Four Corners Power Plant.jpg/credit}}<br/>|}}Flue gas emissions from a [[conventional coal-fired power plant]] in [[New Mexico (U.S. state)|New Mexico]].]] | |||
[[ | '''Flue gas emissions from [[fossil fuel]] combustion ''' refers to the [[emissions]] of [[combustion]] product [[flue gas]] resulting from the burning of [[fossil fuel]]s.<ref name=EPA>[http://www.epa.gov/ttn/chief/ap42/index.html Compilation of Air Pollutant Emission Factors]</ref> | ||
Most fossil fuels are combusted with ambient air (as differentiated from combustion with pure [[oxygen]]) and this article is based on the use of ambient air as the combustion air. | |||
Most fossil fuels are combusted with ambient air (as differentiated from combustion with pure [[oxygen]]) and this article is based on the use of ambient air as the combustion air. | |||
== Discussion of flue gas components== | == Discussion of flue gas components== | ||
Since ambient air contains about 79 volume percent gaseous [[nitrogen]] (N<sub>2</sub>)<ref>{{cite book|author=Perry, R.H. and Green, D.W. (Editors)|title=[[Perry's Chemical Engineers' Handbook]]|edition=7th Edition|publisher=McGraw Hill|year=1997|id=ISBN ISBN 0-07-049841-5}}</ref>, which is essentially non-combustible, the largest part of the [[flue gas]] from most fossil fuel combustion is uncombusted nitrogen. The next largest part of the flue gas is [[carbon dioxide]] (CO<sub>2</sub>) which can be as much as 10 to 15 volume percent or more of the flue gas. This is closely followed in volume by water vapor (H<sub>2</sub>0) created by the combustion of the [[hydrogen]] in the fuel with atmospheric oxygen. Much of the 'smoke' seen exiting from [[flue gas stack]]s is this water vapor forming a fog cloud as it contacts cool air and condenses into water. | |||
Since ambient air contains about 79 volume percent gaseous [[nitrogen]] (N<sub>2</sub>)<ref>{{cite book|author=Perry, R.H. and Green, D.W. (Editors)|title=[[Perry's Chemical Engineers' Handbook]]|edition=7th Edition|publisher=McGraw Hill|year=1997|id=ISBN ISBN 0-07-049841-5}}</ref>, which is essentially non-combustible, the largest part of the [[flue gas]] from most fossil fuel combustion is uncombusted nitrogen. The next largest part of the flue gas is [[carbon dioxide]] (CO<sub>2</sub>) which can be as much as 10 to 15 volume percent or more of the flue gas. This is closely followed in volume by water vapor (H<sub>2</sub>0) created by the combustion of the hydrogen in the fuel with atmospheric oxygen. Much of the 'smoke' seen exiting from [[flue gas stack]]s is this water vapor forming a fog cloud as it contacts cool air and condenses into water. | |||
=== Flue gas pollutants === | === Flue gas pollutants === | ||
A typical flue gas from the combustion of fossil fuels will also contain some very small amounts of [[nitrogen oxide]]s (NOx), [[sulfur dioxide]] (SO<sub>2</sub>) and [[particulate matter]].<ref name=EPA/> The nitrogen oxides are derived from the nitrogen in the ambient air as well as from any nitrogen-containing compounds in the fossil fuel. The sulfur dioxide is derived from any [[sulphur]]-containing compounds in the fuels. The particulate matter is composed of very small particles of solid materials and very small liquid droplets which give flue gases their smoky appearance. | |||
A typical flue gas from the combustion of fossil fuels will also contain some very small amounts of [[nitrogen oxide]]s (NOx), [[sulfur dioxide]] (SO<sub>2</sub>) and [[particulate matter]].<ref name=EPA/> The nitrogen oxides are derived from the nitrogen in the ambient air as well as from any nitrogen-containing compounds in the fossil fuel. The sulfur dioxide is derived from any [[ | |||
== Calculated flue gas emissions from burning fossil fuels == | == Calculated flue gas emissions from burning fossil fuels == | ||
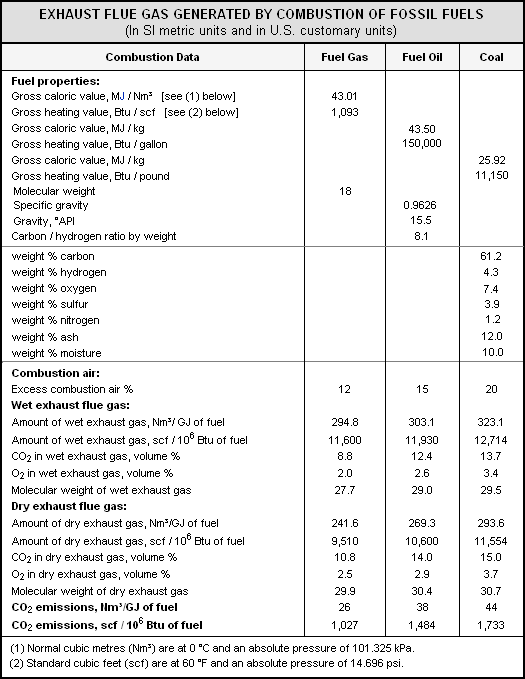
The steam generators in large [[power plant]]s and the process [[furnace]]s in large [[Petroleum refining processes|petroleum refineries]], [[petrochemical]] and [[chemical plant]]s, and [[incinerator]]s burn very considerable amounts of fossil fuels and therefore emit large amounts of flue gas to the ambient atmosphere. The table below presents the total amounts of flue gas typically generated by the burning of fossil fuels such as [[natural gas]], [[fuel oil]] and [[coal]]. The data in the table were obtained by [[stoichometry|stoichiometric]]<ref>{{cite book|author=Zumdahl, Steven S.|title=Chemical Principles|edition=5th Edition|publisher=Houghton Mifflin College Division|year=2005|id=ISBN 0-618-37206-7}}</ref> | The steam generators in large [[power plant]]s and the process [[furnace]]s in large [[Petroleum refining processes|petroleum refineries]], [[petrochemical]] and [[chemical plant]]s, and [[incinerator]]s burn very considerable amounts of fossil fuels and therefore emit large amounts of flue gas to the ambient atmosphere. The table below presents the total amounts of flue gas typically generated by the burning of fossil fuels such as [[natural gas]], [[fuel oil]] and [[coal]]. The data in the table were obtained by [[stoichometry|stoichiometric]]<ref>{{cite book|author=Zumdahl, Steven S.|title=Chemical Principles|edition=5th Edition|publisher=Houghton Mifflin College Division|year=2005|id=ISBN 0-618-37206-7}}</ref> | ||
calculations.<ref>[http://www.air-dispersion.com/formulas.html#combustion Air Dispersion Modeling Conversions and Formulas]</ref> | calculations.<ref>[http://www.air-dispersion.com/formulas.html#combustion Air Dispersion Modeling Conversions and Formulas]</ref> | ||
It is of interest to note that the total amount of flue gas generated by coal combustion is only 10 percent higher than the flue gas generated by natural gas combustion. | It is of interest to note that the total amount of wet flue gas generated by coal combustion is only 10 percent higher than the flue gas generated by natural gas combustion, but the total amount of dry flue gas generated by coal combustion is about 22 percent higher than the flue gas generated by natural gas combustion. This is due to the fact that burning natural gas produces more water vapor than does the burning of coal since the hydrogen-to-[[carbon]] ratio of natural gas is higher than that of coal. | ||
[[ | |||
However, what may be of more interest is that the amount of CO<sub>2</sub> emitted by coal combustion is about 1.7 times the amount emitted by natural gas combustion. | |||
{{Image|FossilFuelFlueGas.png|center|525px}} | |||
==References== | ==References== | ||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
{{reflist}} | |||
[[Category: | |||
Latest revision as of 11:03, 17 August 2024

Flue gas emissions from a conventional coal-fired power plant in New Mexico.
Flue gas emissions from fossil fuel combustion refers to the emissions of combustion product flue gas resulting from the burning of fossil fuels.[1]
Most fossil fuels are combusted with ambient air (as differentiated from combustion with pure oxygen) and this article is based on the use of ambient air as the combustion air.
Discussion of flue gas components
Since ambient air contains about 79 volume percent gaseous nitrogen (N2)[2], which is essentially non-combustible, the largest part of the flue gas from most fossil fuel combustion is uncombusted nitrogen. The next largest part of the flue gas is carbon dioxide (CO2) which can be as much as 10 to 15 volume percent or more of the flue gas. This is closely followed in volume by water vapor (H20) created by the combustion of the hydrogen in the fuel with atmospheric oxygen. Much of the 'smoke' seen exiting from flue gas stacks is this water vapor forming a fog cloud as it contacts cool air and condenses into water.
Flue gas pollutants
A typical flue gas from the combustion of fossil fuels will also contain some very small amounts of nitrogen oxides (NOx), sulfur dioxide (SO2) and particulate matter.[1] The nitrogen oxides are derived from the nitrogen in the ambient air as well as from any nitrogen-containing compounds in the fossil fuel. The sulfur dioxide is derived from any sulphur-containing compounds in the fuels. The particulate matter is composed of very small particles of solid materials and very small liquid droplets which give flue gases their smoky appearance.
Calculated flue gas emissions from burning fossil fuels
The steam generators in large power plants and the process furnaces in large petroleum refineries, petrochemical and chemical plants, and incinerators burn very considerable amounts of fossil fuels and therefore emit large amounts of flue gas to the ambient atmosphere. The table below presents the total amounts of flue gas typically generated by the burning of fossil fuels such as natural gas, fuel oil and coal. The data in the table were obtained by stoichiometric[3] calculations.[4]
It is of interest to note that the total amount of wet flue gas generated by coal combustion is only 10 percent higher than the flue gas generated by natural gas combustion, but the total amount of dry flue gas generated by coal combustion is about 22 percent higher than the flue gas generated by natural gas combustion. This is due to the fact that burning natural gas produces more water vapor than does the burning of coal since the hydrogen-to-carbon ratio of natural gas is higher than that of coal.
However, what may be of more interest is that the amount of CO2 emitted by coal combustion is about 1.7 times the amount emitted by natural gas combustion.
References
- ↑ 1.0 1.1 Compilation of Air Pollutant Emission Factors
- ↑ Perry, R.H. and Green, D.W. (Editors) (1997). Perry's Chemical Engineers' Handbook, 7th Edition. McGraw Hill. ISBN ISBN 0-07-049841-5.
- ↑ Zumdahl, Steven S. (2005). Chemical Principles, 5th Edition. Houghton Mifflin College Division. ISBN 0-618-37206-7.
- ↑ Air Dispersion Modeling Conversions and Formulas