Amoxicillin: Difference between revisions
imported>David E. Volk mNo edit summary |
mNo edit summary |
||
| (9 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC|left}} | |||
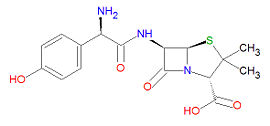
'''Amoxicillin''' ('''AMX'''), also spelled '''amoxycillin''', and also called '''p-hydroxyampicillin''', is an [[antibiotic]] drug that is very similar to [[ampicillin]] but which has enhanced stability towards gastric juices compared to other <math>\beta</math>-lactam antibiotics. It is effective against a wide range of | {{Chem infobox | ||
|align=right | |||
|image=[[Image:Amoxicillin structure.jpg|center|thumb|270px|{{#ifexist:Template:Amoxicillin structure.jpg/credit|{{Amoxicillin structure.jpg/credit}}<br/>|}}]] | |||
|width=270px | |||
|molname=amoxicillin | |||
|synonyms='''AMX''', '''p-hydroxyampicillin''' | |||
|molformula= C<sub>16</sub>H<sub>19</sub>N<sub>3</sub>O<sub>5</sub>S | |||
|molmass= 365.4042 | |||
|uses=antibiotic drug | |||
|properties=beta-lactam | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=26787-78-0 | |||
}} | |||
'''Amoxicillin''' ('''AMX'''), also spelled '''amoxycillin''', and also called '''p-hydroxyampicillin''', is an [[antibiotic]] drug that is very similar to [[ampicillin]] but which has enhanced stability towards gastric juices compared to other <math>\beta</math>-lactam antibiotics. It is effective against a wide range of Gram-positive bacteria but limited Gram-negative bacteria. It is used for bacterial strains that do not produce <math>\beta</math>-[[lactamase]], an enzyme which would degrade the drug. The incidence of <math>\beta</math>-lactamase-resistance in bacteria is increasing, leading to the increased use of [[clavulanic acid]], a <math>\beta</math>-lactamase inhibitor, with amoxicillin and other <math>\beta</math>-lactam drugs. It is used to treat infections in the ears, nose and throat, the genitourinary tract, the skin and the respiratory tract. It is effective against [[E. coli]], [[S. pneumoniae]], [[Staphylococcus spp.]], [[H. influenzae]], [[P. mirabilis]], [[E. faecalis]], [[N. gonorrheae]] and some strains of [[Streptococcus spp.]]. | |||
== Chemical properties == | == Chemical properties == | ||
Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and its chemical formula is C<sub>16</sub>H<sub>19</sub>N<sub>3</sub>O<sub>5</sub>S. It is a beta-lactam drug. | Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and its chemical formula is C<sub>16</sub>H<sub>19</sub>N<sub>3</sub>O<sub>5</sub>S, giving it a molecular mass of 365.4042 g/mol. It is a beta-lactam drug and is hence susceptible to degradation by <math>\beta</math>-[[lactamase]] enzymes. | ||
== Mechanism of action == | == Mechanism of action == | ||
| Line 14: | Line 28: | ||
== Drug interactions == | == Drug interactions == | ||
Amoxicillin may be an agonist of [[demeclycycline]], [[doxycycline]], [[methacycline]], [[minocycline]], [[oxytetracycline]], [[Rolitetracycline]] and [[tetracycline]]. It may decrease the effects of some contraceptives, including [[ethinyl estradiol]] and [[mestranol]], and increases the effects of [[methotrexate]]. | Amoxicillin may be an agonist of [[demeclycycline]], [[doxycycline]], [[methacycline]], [[minocycline]], [[oxytetracycline]], [[Rolitetracycline]] and [[tetracycline]]. It may decrease the effects of some contraceptives, including [[ethinyl estradiol]] and [[mestranol]], and increases the effects of [[methotrexate]]. | ||
== Synonyms == | |||
{{col-begin}} | |||
{{col-break|width=33%}} | |||
*Amoxicilina | |||
*Amoxicillin | |||
*Amoxicillin | |||
*Amoxicilline | |||
*Amoxicillinum | |||
{{col-break|width=33%}} | |||
*AMC | |||
*Amoxycillin Trihydrate | |||
*Amoxycillin | |||
*D-Amoxicillin | |||
*p-Hydroxyampicillin | |||
{{col-break|width=33%}} | |||
{{col-end}} | |||
== Brand names == | |||
{{col-begin}} | |||
{{col-break|width=33%}} | |||
*AMPC® | |||
*Actimoxi® | |||
*Amoclen® | |||
*Amolin® | |||
*Amopen® | |||
*Amopenixin® | |||
*Amoxi® | |||
*Amoxi-Mast® | |||
*Amoxibiotic® | |||
*Amoxiden® | |||
*Amoxil® | |||
*Amoxivet® | |||
*Anemolin® | |||
*Aspenil® | |||
*Biomox® | |||
{{col-break|width=33%}} | |||
*Bristamox® | |||
*Cemoxin® | |||
*Clamoxyl® | |||
*Delacillin® | |||
*Dispermox® | |||
*Efpenix® | |||
*Flemoxin® | |||
*Hiconcil® | |||
*Histocillin® | |||
*Ibiamox® | |||
*Imacillin® | |||
*Lamoxy® | |||
*Metafarma® capsules | |||
*Metifarma® capsules | |||
*Moxacin® | |||
{{col-break|width=33%}} | |||
*Moxal® | |||
*Ospamox® | |||
*Pamoxicillin® | |||
*Piramox® | |||
*Polymox® | |||
*Robamox® | |||
*Sawamox® PM | |||
*Sumox® | |||
*Tolodina® | |||
*Trimox® | |||
*Unicillin® | |||
*Utimox® | |||
*Vetramox® | |||
*Wymox® | |||
*Zimox® | |||
{{col-break|width=33%}} | |||
{{col-end}} | |||
== External links == | == External links == | ||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 10 July 2024
|
| |||||||
| amoxicillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Amoxicillin (AMX), also spelled amoxycillin, and also called p-hydroxyampicillin, is an antibiotic drug that is very similar to ampicillin but which has enhanced stability towards gastric juices compared to other -lactam antibiotics. It is effective against a wide range of Gram-positive bacteria but limited Gram-negative bacteria. It is used for bacterial strains that do not produce -lactamase, an enzyme which would degrade the drug. The incidence of -lactamase-resistance in bacteria is increasing, leading to the increased use of clavulanic acid, a -lactamase inhibitor, with amoxicillin and other -lactam drugs. It is used to treat infections in the ears, nose and throat, the genitourinary tract, the skin and the respiratory tract. It is effective against E. coli, S. pneumoniae, Staphylococcus spp., H. influenzae, P. mirabilis, E. faecalis, N. gonorrheae and some strains of Streptococcus spp..
Chemical properties
Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and its chemical formula is C16H19N3O5S, giving it a molecular mass of 365.4042 g/mol. It is a beta-lactam drug and is hence susceptible to degradation by -lactamase enzymes.
Mechanism of action
Amoxicillin binds to the penicillin-binding protein 1A (PBP-1A) located inside the bacterial cell well. It inhibits the last stage of bacterial cell wall synthesis by acylating the penicillin-sensitive transpeptidase C-terminal domain thereby stopping the cross-linking of peptidoglycan strands. Autolytic enzymes in the bacteria then lyse the cells.
Drug interactions
Amoxicillin may be an agonist of demeclycycline, doxycycline, methacycline, minocycline, oxytetracycline, Rolitetracycline and tetracycline. It may decrease the effects of some contraceptives, including ethinyl estradiol and mestranol, and increases the effects of methotrexate.
Synonyms
|
|
Brand names
|
|
|
External links
The most up-to-date information about Amoxicillin and other drugs can be found at the following sites.
- Amoxicillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Amoxicillin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Amoxicillin - Detailed information from DrugBank.