Penciclovir: Difference between revisions
imported>David E. Volk m (→External links) |
mNo edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Penciclovir structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Penciclovir structure.jpg|center|thumb|250px]] | |||
|width=250px | |||
|molname=penciclovir | |||
|synonyms= '''Denavir®, PE2, penciclovirum''' | |||
|molformula= C<sub>10</sub>H<sub>15</sub>N<sub>5</sub>O<sub>3</sub> | |||
|molmass= 253.2578 | |||
|uses=herpes viruses | |||
|properties=guanosine analog | |||
|hazards=see drug interactions | |||
|iupac= see chemistry | |||
|casnumber=84625-61-6 | |||
}} | |||
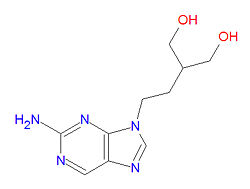
'''Penciclovir''' ('''PE2''') or '''penciclovirum''', is a [[guanosine]] analog, [[antiviral drug]] used to treat [[herpesvirus]]es and is the active metabolite of the prodrug [[famciclovir]]. It is sold under the brand name '''Denavir®'''. The longer [[half-life]] of penciclovir triphosphate in herpes-infected cells (10-20 hrs), compared to that of [[acyclovir]] (0,7 -1 hr), may be responsible for its ehanced results. | '''Penciclovir''' ('''PE2''') or '''penciclovirum''', is a [[guanosine]] analog, [[antiviral drug]] used to treat [[herpesvirus]]es and is the active metabolite of the prodrug [[famciclovir]]. It is sold under the brand name '''Denavir®'''. The longer [[half-life]] of penciclovir triphosphate in herpes-infected cells (10-20 hrs), compared to that of [[acyclovir]] (0,7 -1 hr), may be responsible for its ehanced results. | ||
The triphosphate form competes with [[deoxyguanosine]] triphosphate for incorporation into viral DNA. It only effects DNA synthesis of only those cells infected with [[herpes simplex virus]] (HSV). | The triphosphate form competes with [[deoxyguanosine]] triphosphate for incorporation into viral DNA. It only effects DNA synthesis of only those cells infected with [[herpes simplex virus]] (HSV). | ||
== Chemistry == | |||
The IUPAC chemical name of penciclovir is 2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-3H-purin-6-one, and it has chemical formula C<sub>10</sub>H<sub>15</sub>N<sub>5</sub>O<sub>3</sub>, giving it a molecular mass of 253.2578 g/mol. It is an analog of the natural nucleotide guanosine. The prodrug famciclovir is an analog of this drug. | |||
== External links == | == External links == | ||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:01, 2 October 2024
|
| |||||||
| penciclovir | |||||||
| |||||||
| Uses: | herpes viruses | ||||||
| Properties: | guanosine analog | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Penciclovir (PE2) or penciclovirum, is a guanosine analog, antiviral drug used to treat herpesviruses and is the active metabolite of the prodrug famciclovir. It is sold under the brand name Denavir®. The longer half-life of penciclovir triphosphate in herpes-infected cells (10-20 hrs), compared to that of acyclovir (0,7 -1 hr), may be responsible for its ehanced results. The triphosphate form competes with deoxyguanosine triphosphate for incorporation into viral DNA. It only effects DNA synthesis of only those cells infected with herpes simplex virus (HSV).
Chemistry
The IUPAC chemical name of penciclovir is 2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-3H-purin-6-one, and it has chemical formula C10H15N5O3, giving it a molecular mass of 253.2578 g/mol. It is an analog of the natural nucleotide guanosine. The prodrug famciclovir is an analog of this drug.
External links
The most up-to-date information about Penciclovir and other drugs can be found at the following sites.
- Penciclovir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Penciclovir - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Penciclovir - Detailed information from DrugBank.