Didanosine: Difference between revisions
imported>David E. Volk mNo edit summary |
mNo edit summary |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 17: | Line 17: | ||
'''Didanosine''' ('''DDI'''), or '''dideoxyinosine''', is a potent [[HIV]]-1 [[reverse transcriptase inhibitor]] that also acts as a viral [[DNA]] chain terminator. Treatment with DDI after long-term [[AZT]] treatment has been beneficial. Didanosine is unusual among nucleoside analogs because it does not contain a regular base or regular sugar, but instead contains hypoxanthine attached to a dideoxy sugar. The absence of a 3'-hydroxy group on the sugar prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains. DDI is metabolized to dideoxyadenosine triphosphate (ddATP), its putative active metabolite that competes with dATP. It is often taken with antacids because its stability is low under acidic conditions. It is sold under the brand names '''Videx®''' and '''Videx® EC'''. Possible toxic side effects including [[diarrhea]], [[hepatic dysfunction|liver dysfunction]], [[hyperuricemia]], [[pancreatitis]] and peripheral [[neuropathy]]. | '''Didanosine''' ('''DDI'''), or '''dideoxyinosine''', is a potent [[HIV]]-1 [[reverse transcriptase inhibitor]] that also acts as a viral [[DNA]] chain terminator. Treatment with DDI after long-term [[AZT]] treatment has been beneficial. Didanosine is unusual among nucleoside analogs because it does not contain a regular base or regular sugar, but instead contains hypoxanthine attached to a dideoxy sugar. The absence of a 3'-hydroxy group on the sugar prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains. DDI is metabolized to dideoxyadenosine triphosphate (ddATP), its putative active metabolite that competes with dATP. It is often taken with antacids because its stability is low under acidic conditions. It is sold under the brand names '''Videx®''' and '''Videx® EC'''. Possible toxic side effects including [[diarrhea]], [[hepatic dysfunction|liver dysfunction]], [[hyperuricemia]], [[pancreatitis]] and peripheral [[neuropathy]]. | ||
== Chemistry == | |||
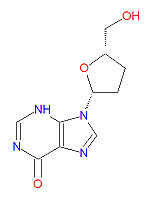
Its IUPAC chemical name is 9-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one and its chemical formula is C<sub>10</sub>H<sub>12</sub>N<sub>4</sub>O<sub>3</sub> (MW 236.2273). | |||
== Drug Interactions == | |||
The effects and toxicity of didanosine is increased when taken with [[ganciclovir]], [[tenofovir]] or [[valganciclovir]]. DDI levels may be reduced when taken with [[tipranavir]]. The risk of peripheral neuropathy toxicity is additive for the DDI/[[zalcitabine]] combination. | |||
== External Links == | |||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 7 August 2024
|
| |||||||
| didanosine | |||||||
| |||||||
| Uses: | HIV | ||||||
| Properties: | Reverse transcriptase inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Didanosine (DDI), or dideoxyinosine, is a potent HIV-1 reverse transcriptase inhibitor that also acts as a viral DNA chain terminator. Treatment with DDI after long-term AZT treatment has been beneficial. Didanosine is unusual among nucleoside analogs because it does not contain a regular base or regular sugar, but instead contains hypoxanthine attached to a dideoxy sugar. The absence of a 3'-hydroxy group on the sugar prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains. DDI is metabolized to dideoxyadenosine triphosphate (ddATP), its putative active metabolite that competes with dATP. It is often taken with antacids because its stability is low under acidic conditions. It is sold under the brand names Videx® and Videx® EC. Possible toxic side effects including diarrhea, liver dysfunction, hyperuricemia, pancreatitis and peripheral neuropathy.
Chemistry
Its IUPAC chemical name is 9-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one and its chemical formula is C10H12N4O3 (MW 236.2273).
Drug Interactions
The effects and toxicity of didanosine is increased when taken with ganciclovir, tenofovir or valganciclovir. DDI levels may be reduced when taken with tipranavir. The risk of peripheral neuropathy toxicity is additive for the DDI/zalcitabine combination.
External Links
The most up-to-date information about Didanosine and other drugs can be found at the following sites.
- Didanosine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Didanosine - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Didanosine - Detailed information from DrugBank.