Mevalonate: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (stub and structure) |
mNo edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
[[Image:Mevalonate structure.jpg|right|thumb|150px|{{|{{Mevalonate structure.jpg/credit}}<br/>|Mevalonate.]] | [[Image:Mevalonate structure.jpg|right|thumb|150px|{{|{{Mevalonate structure.jpg/credit}}<br/>|Mevalonate.]] | ||
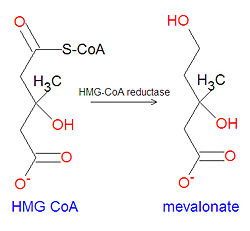
'''Mevalonate''' is a key chemical precursor in the biosynthesis of [[cholesterol]]. The statin drugs used to lower cholesterol are [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitor]]s that work by inhibiting the synthesis of mevalonate from [[3-hydroxy-3-methyl-glutaryl CoA]] (HMG-CoA). | '''Mevalonate''' is a key chemical precursor in the biosynthesis of [[cholesterol]]. The statin drugs used to lower cholesterol are [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitor]]s that work by inhibiting the synthesis of mevalonate from the reduction of [[3-hydroxy-3-methyl-glutaryl CoA]] (HMG-CoA). | ||
{{Image|Mevalonate synthesis.jpg|left|250px|Biosynthesis of mevalonate from HMG CoA.}} | |||
Its IUPAC chemical name is (3R)-3,5-dihydroxy-3-methylpentanoic acid and its chemical formula is C<sub>6</sub>H<sub>12</sub>O<small>4</small> in the protonated state.[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:01, 18 September 2024
Mevalonate is a key chemical precursor in the biosynthesis of cholesterol. The statin drugs used to lower cholesterol are HMG-CoA reductase inhibitors that work by inhibiting the synthesis of mevalonate from the reduction of 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA).
Its IUPAC chemical name is (3R)-3,5-dihydroxy-3-methylpentanoic acid and its chemical formula is C6H12O4 in the protonated state.