Methane: Difference between revisions
imported>David E. Volk (remove cats) |
mNo edit summary |
||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
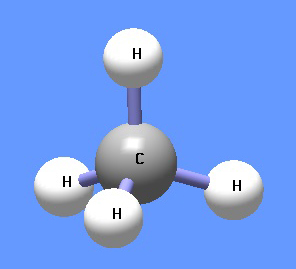
{{Image|Methane DEVolk.jpg|right|350px|Methane.}} | |||
'''Methane''' (CH<sub>4</sub>) is under normal temperature and pressure a colorless and odorless gas, also known as marsh gas. It is lighter than air, boils at −161.4 <sup>0</sup>C and solidifies at | '''Methane''' (CH<sub>4</sub>) is under normal temperature and pressure a colorless and odorless gas, also known as marsh gas. It is lighter than air, boils at −161.4 <sup>0</sup>C and solidifies at | ||
−184 <sup>0</sup>C. It is highly combustible and forms an explosive mixture with air. The gas is formed by decomposition of plant and animal matter and is the principal component of natural gas and fire damp in coal mines. Methane is used in the heating of homes and the industrial preparation of [[hydrogen]]. In [[chemistry]], it is the first member of a series of saturated hydrocarbons, the [[alkanes]]. Methane is a potent [[greenhouse gas]]. | −184 <sup>0</sup>C. It is highly combustible and forms an [[Explosives|explosive]] mixture with air. The gas is formed by decomposition of plant and animal matter and is the principal component of natural gas and fire damp in coal mines. Methane is used in the heating of homes and the industrial preparation of [[hydrogen]]. In [[chemistry]], it is the first member of a series of saturated hydrocarbons, the [[alkanes]]. Methane is a potent [[greenhouse gas]]. Large deposits of methane are believed to be contained [[clathrate hydrates]] along ocean floors as well as under [[permafrost]], far exceeding reserves of conventional [[natural gas]].<ref>[http://geology.usgs.gov/connections/mms/joint_projects/methane.htm U.S. Geological Survey, Methane Gas Hydrates]</ref> [[Global warming]] could release this gas and thus enhance considerably the greenhouse effect. | ||
==References== | |||
{{Reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 18 September 2024
Methane (CH4) is under normal temperature and pressure a colorless and odorless gas, also known as marsh gas. It is lighter than air, boils at −161.4 0C and solidifies at −184 0C. It is highly combustible and forms an explosive mixture with air. The gas is formed by decomposition of plant and animal matter and is the principal component of natural gas and fire damp in coal mines. Methane is used in the heating of homes and the industrial preparation of hydrogen. In chemistry, it is the first member of a series of saturated hydrocarbons, the alkanes. Methane is a potent greenhouse gas. Large deposits of methane are believed to be contained clathrate hydrates along ocean floors as well as under permafrost, far exceeding reserves of conventional natural gas.[1] Global warming could release this gas and thus enhance considerably the greenhouse effect.