Alzheimer's disease: Difference between revisions
imported>Matt Lewis (→Research: grammar) |
m (→Diagnosis: adding the mention of PET scan) |
||
| (49 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC|left}} | |||
'''Alzheimer's disease''' is also known as '''Alzheimer disease''', '''Alzheimer's''' and simply '''AD''' | {{Infobox_Disease | | ||
Name = Alzheimer's disease | | |||
Image = Alzheimers_disease_-_MRI.jpg|thumb| | |||
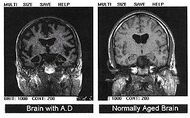
Caption = Post-autopsy brain scans: A brain with Alzheimer's disease (left) as compared to a normal brain (right)| | |||
| DiseasesDB = 490 | |||
| ICD10 = {{ICD10|G|30||g|30}}, {{ICD10|F|00||f|00}} | |||
| ICD9 = {{ICD9|331.0}}-{{ICD9|290.1}} | |||
| OMIM = 104300 | |||
| MedlinePlus = 000760 | |||
}} | |||
'''Alzheimer's disease''' is also known as '''Alzheimer disease''', '''Alzheimer's''' and simply '''AD'''. | |||
Alzheimer's the most common cause of [[dementia]], afflicting | Alzheimer's is the most common cause of [[dementia]], afflicting at least 44 million people worldwide, as of 2016. Alzheimer's is a [[terminal disease]] for which there is currently no known cure. It is most commonly found in people over 65 years old, although a less-common form called [[Familial Alzheimer's disease]], or "early-onset Alzheimer's", also occurs, affecting about 1%—5% of the total of Alzheimer's sufferers.<ref name="pmid16360788"> | ||
{{cite journal | {{cite journal | ||
|author=Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M | |author=Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M | ||
| Line 17: | Line 27: | ||
}}</ref> | }}</ref> | ||
Typically, the disease begins many years before it is diagnosed. In its early stages, | Typically, the disease begins many years before it is diagnosed. In its early stages, short-term memory loss is the most common symptom, which is often initially thought by the sufferer to be caused by other factors, such as aging or stress.<ref name="alzdiag"> | ||
{{cite journal | {{cite journal | ||
|author=Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B | |author=Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B | ||
| Line 29: | Line 39: | ||
|doi=10.1111/j.1468-1331.2006.01605.x | |doi=10.1111/j.1468-1331.2006.01605.x | ||
}}</ref> | }}</ref> | ||
Later symptoms of the disease include confusion, anger, mood swings, language breakdown, | Later symptoms of the disease include confusion, anger, mood swings, language breakdown, long-term memory loss, and the general "withdrawal" of the sufferer as his or her senses decline.<ref name="alzdiag"/><ref name="pmid17823840"> | ||
{{cite journal | {{cite journal | ||
|author=Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP | |author=Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP | ||
| Line 64: | Line 74: | ||
}}</ref> | }}</ref> | ||
==Symptoms== | |||
Common symptoms of dementia include: | |||
A decline in memory | |||
Changes in thinking skills | |||
Poor judgment and reasoning | |||
Decreased focus and attention | |||
Decreased language ability | |||
Negative changes in behavior | |||
Although the symptoms are common, they are typically experienced in unique ways.<ref name="alzheimers.org"> | Although the symptoms are common, they are typically experienced in unique ways.<ref name="alzheimers.org"> | ||
| Line 76: | Line 94: | ||
"Stages" are commonly referred to by professionals to describe the [[progressive disease|progressive]] nature of Alzheimer's (typically "early", "mid" and "late onset") but the symptoms can cross over these "boundaries" for many sufferers. | "Stages" are commonly referred to by professionals to describe the [[progressive disease|progressive]] nature of Alzheimer's (typically "early", "mid" and "late onset") but the symptoms can cross over these "boundaries" for many sufferers. | ||
==Diagnosis== | |||
The symptoms of Alzheimer's disease are generally reported to a doctor or physician when memory-loss (or symptoms surrounding memory loss) begins to pose a serious concern. When Alzheimer's disease is suspected, diagnosis is typically confirmed by a behavioural assessment, and some form of cognitive test. Often this is followed by a [[brain scan]], such as a PET scan.<ref name="alzres"> | |||
The symptoms of Alzheimer's disease are generally reported to a doctor or physician when memory-loss (or symptoms surrounding memory loss) | |||
{{cite web | {{cite web | ||
| title=Alzheimer's Diagnosis of AD | | title=Alzheimer's Diagnosis of AD | ||
| Line 86: | Line 103: | ||
}}</ref> | }}</ref> | ||
=== | ===Criteria=== | ||
There are three sets of criteria for the clinical diagnoses of the spectrum of Alzheimer's disease: the 2013 fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5); the National Institute on Aging-Alzheimer's Association (NIA-AA) definition as revised in 2011; and the International Working Group criteria as revised in 2010. Three periods, which can span decades, define the progression of Alzheimer's disease from the preclinical phase, to mild cognitive impairment (MCI), followed by Alzheimer's disease dementia. | |||
Eight intellectual domains are most commonly impaired in AD: memory, language, perceptual skills, attention, motor skills, orientation, problem solving, and executive functional abilities, as listed in the fourth text revision of the DSM (DSM-IV-TR). | |||
The DSM-5 defines criteria for probable or possible Alzheimer's for both major and mild neurocognitive disorder. Major or mild neurocognitive disorder must be present along with at least one cognitive deficit for a diagnosis of either probable or possible AD. | |||
===Laboratory tests=== | |||
====Apolipoprotein E4==== | |||
Although [[apolipoprotein E4]] is an important susceptibility gene for Alzheimer's disease]<ref name="pmid10944568">{{cite journal |author=Skoog I |title=Detection of preclinical Alzheimer's disease |journal=N. Engl. J. Med. |volume=343 |issue=7 |pages=502–3 |year=2000 |month=August |pmid=10944568 |doi= |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=10944568&promo=ONFLNS19 |issn= |quote=The APOE 4 allele is a susceptibility gene for Alzheimer's disease and seems to affect the age of onset of the disease. However, the presence of this allele alone is not sufficient to predict which asymptomatic subjects will ultimately have Alzheimer's disease, and the disease never develops in many subjects with this genotype}}</ref>, its [[sensitivity and specificity]] are insufficient (65 and 68 percent, respectively) to be used as a diagnostic test.<ref name="pmid12160362">{{cite journal |author=Kivipelto M, Helkala EL, Laakso MP, ''et al'' |title=Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease |journal=Ann. Intern. Med. |volume=137 |issue=3 |pages=149–55 |year=2002 |month=August |pmid=12160362 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=reprint&pmid=12160362 |issn=}}</ref> | |||
However, the level of [[apolipoprotein E4]] in [[cerebrospinal fluid]] may be predictive.<ref name="pmid20697045">{{cite journal| author=De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP et al.| title=Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. | journal=Arch Neurol | year= 2010 | volume= 67 | issue= 8 | pages= 949-56 | pmid=20697045 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20697045 | doi=10.1001/archneurol.2010.179 }} </ref> | |||
====Amyloid-beta protein==== | |||
Amyloid-beta protein may be elevated in the cerebrospinal fluid of some patients.<ref name="pmid20697045" /> | |||
===Autopsy=== | |||
Completely reliable confirmation of a Alzheimer's diagnosis has traditionally only been possible upon the death of the patient. The brain tissue will be examined by a pathologist, and there are two unique lesions that are unmistakable signs of Alzheimer's that will give definite confirmation. | |||
==Cause/etiology== | |||
The etiology of Alzheimer's disease is incompletely understood. Alzheimer's disease is associated with senile plaques and neurofibrillary tangles in the [[brain]].<ref name="pmid15184601">{{cite journal |author=Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J |title=The importance of neuritic plaques and tangles to the development and evolution of AD |journal=Neurology |volume=62 |issue=11 | |||
|pages=1984–1989 |year=2004 |pmid=15184601 |doi=}}</ref> The incorrect folding of [[protein]]s leads to the formation of amyloid plaques. The prion protein (PrP<sup>C</sup>) may be the cellular receptor for amyloid-beta oligomer.<ref name="pmid19242475">{{cite journal |author=Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM |title=Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers |journal=Nature |volume=457 |issue=7233 |pages=1128–32 |year=2009 |month=February |pmid=19242475 |doi=10.1038/nature07761 |url=http://dx.doi.org/10.1038/nature07761 |issn=}}</ref> Current research aims to determine if such plaques are the result of, or the cause of, Alzheimer's disease. | |||
Regarding biomarkers, one study found that "a reduction in the cerebrospinal fluid (CSF) Aβ42 level denotes a pathophysiological process that significantly departs from normality (i.e., becomes dynamic) early, whereas the CSF total tau level and the adjusted hippocampal volume are biomarkers of downstream pathophysiological processes". <ref name="pmid21825215">{{cite journal| author=Jack CR, Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ et al.| title=Evidence for ordering of Alzheimer disease biomarkers. | journal=Arch Neurol | year= 2011 | volume= 68 | issue= 12 | pages= 1526-35 | pmid=21825215 | doi=10.1001/archneurol.2011.183 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21825215 }} </ref> | |||
The | Although there is a genetic susceptibility in a small percentage of people, Alzheimer's is not considered a genetic illness. The greatest risk factor for developing Alzheimer's is age, as the percentage of people developing it dramatically increases after age 65 and beyond. Major environmental/lifestyle risk factors include a diet high in saturated fats that are typical of the standard western diet, smoking, and long-term exposure to environmental toxins such as aluminium. Research is ongoing. | ||
Alzheimer's appears to be a disease of the modern world, especially developed societies. Prior to the early 20th century, this dementia was completely unknown to science and was never described in elder members of traditional societies. | |||
Alzheimer's | Individuals with the genetic disease Down syndrome (trisomy 21) are at much higher risk for developing Alzheimer's in their middle age. According to the National Down Syndrome Society, about 30% of people with Down syndrome who are in their 50s, and about 50% of those in their 60s, have Alzheimer’s disease. The lifetime risk is over 90%. <ref> National Down Syndrome Society. Alzheimer’s Disease and | ||
Down Syndrome. Available at: https://www.ndss.org/resources/ | |||
alzheimers/. Accessed July 11, 2024</ref> | |||
=== | == Epidemiology == | ||
In the United States in 2020, Alzheimer's dementia prevalence was estimated to be 5% for those in the 60–74 age group, with the rate increasing to 14% in the 74–84 group and to 35% in those greater than 85.<ref>{{cite journal |vauthors=Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA |title=Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020-2060) |journal=Alzheimer's & Dementia |date=May 2021 |volume=17 |issue=12 |pages=1966–1975 |pmid=34043283 |doi=10.1002/alz.12362 |pmc=9013315 |s2cid=235215290 }}</ref> | |||
Advancing age is a primary risk factor for the disease and incidence rates are not equal for all ages: every 5 years after the age of 65, the risk of acquiring the disease approximately doubles, in developed societies such as Spain and Italy. | |||
==Treatment== | |||
As of 2024, no treatment has been found to stop progression or completely reverse the disease, but some treatments do slow the progression. Many preventive measures have been suggested for Alzheimer's disease, but their values are uncertain: mental stimulation, [[exercise]], and a Mediterranean style diet are usually recommended, both as possible prevention and as a sensible way of managing the disease.<ref name="prevention"> | |||
{{cite web | {{cite web | ||
| title=The Search for AD Prevention Strategies | | title=The Search for AD Prevention Strategies | ||
| Line 114: | Line 151: | ||
}}</ref> | }}</ref> | ||
=== | ===Medications=== | ||
As of 2023, available medications offer relatively small symptomatic benefit for some patients but generally do not slow disease progression. Newer monoclonal antibody treatments against beta-amyloid protein, for example, the drug Leqembi, have been approved in the United States, but they only slightly reduce the rate of disease progression and have significant side effects, including brain bleeding and in rare cases, death. These drugs cost over $20,000 a year, which could be prohibitively expensive for many patients. | |||
[[Randomized controlled trial]]s showed either small or absent benefit from acetylcholinesterase inhibitors<ref> | |||
{{cite journal | {{cite journal | author = Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H, van den Bussche H | title = Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. | journal = BMJ | ||
| author = | | volume = 331 | issue = 7512 | pages = 321-7 | year = 2005 | id = PMID 16081444}} </ref> such as donepezil.<ref name="pmid17914039"> {{cite journal |author=Howard RJ, Juszczak E, Ballard CG, ''et al'' |title=Donepezil for the treatment of agitation in Alzheimer's disease |journal=N. Engl. J. Med. |volume=357 |issue=14 |pages=1382–92 |year=2007 |pmid=17914039 | ||
| title = | |doi=10.1056/NEJMoa066583}}</ref><ref>{{cite journal | author = Courtney C, '''Farrell D''', Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, Lendon C, Shaw H, Bentham P | title = Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. | journal = Lancet | volume = 363 | ||
| journal = | | issue = 9427 | pages = 2105-15 | year = 2004 | id = PMID 15220031}}</ref><ref name="doi10.1056/NEJMoa1106668">Donepezil and Memantine for Moderate-to-Severe Alzheimer's Disease. NEJM 2012. http://dx.doi.org/10.1056/NEJMoa1106668</ref> | ||
| pages = | |||
| id = PMID | |||
==== | The [[N-methyl-d-aspartate receptor]] antagonist memantine has shown some effectiveness<ref name="Areosa"> | ||
{{cite journal | author = Areosa Sastre A, McShane R, Sherriff F | title = Memantine for dementia. | |||
| journal = Cochrane Database Syst Rev | pages = CD003154 | id = PMID 15495043}}</ref> but does not add benefit to donepezil .<ref name="doi10.1056/NEJMoa1106668"/> | |||
Due to the incurable and degenerative nature of the disease care-management of Alzheimer's is essential. The role of the main | === Care management === | ||
Due to the incurable and degenerative nature of the disease care-management of Alzheimer's is essential. The role of the main caregiver is often taken by the spouse or a close relative.<ref name="glam"> | |||
{{cite journal | {{cite journal | ||
|url=http://www.cncforum.me.uk/S.O'Donovan%20PhD%20Thesis%20Exec%20Summary%202004.pdf | |url=http://www.cncforum.me.uk/S.O'Donovan%20PhD%20Thesis%20Exec%20Summary%202004.pdf | ||
| Line 200: | Line 187: | ||
}}</ref> | }}</ref> | ||
== Alzheimer's in society == | |||
Famous people who have, or have died of Alzheimer's disease, are the [[president of the United States of America|US president]] [[Ronald Reagan]], the [[Prime Minister of the United Kingdom| UK Prime minister]] [[Harold Wilson]], the writers [[Terry Pratchett]] and Iris Murdoch, and the film stars [[Rita Hayworth]] and Charlton Heston. | |||
== History == | |||
[[Image:593px-Auguste D aus Marktbreit.jpg|thumb|[[Auguste D]], first recorded patient with the newly recognised form of dementia.]] | |||
Alzheimer’s disease is named after Dr. Alois Alzheimer, the German physician who first described it. In 1901, Alzheimer observed a 51-year-old patient at the Frankfurt asylum named Auguste Deter. Her symptoms included memory loss, language problems, and unpredictable behavior. In 1906, she died and Dr. Alzheimer noticed unusual pathology in her brain tissue; he found many abnormal clumps (now called amyloid plaques) and tangled bundles of fibers (now called neurofibrillary, or tau, tangles). | |||
Prior to the early 20th century, Alzheimer's was undescribed in world medical literature. Other dementias and brain and behavioral disorders, however, were well documented by then. | |||
==References== | ==References== | ||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 17:09, 12 July 2024
| Alzheimer's disease | |
|---|---|
| Post-autopsy brain scans: A brain with Alzheimer's disease (left) as compared to a normal brain (right) | |
| ICD-10 | ICD10 F84.0-F84.1, ICD10 F84.0-F84.1 |
| ICD-9 | 331.0 |
| OMIM | 104300 |
| MedlinePlus | 000760 |
Alzheimer's disease is also known as Alzheimer disease, Alzheimer's and simply AD.
Alzheimer's is the most common cause of dementia, afflicting at least 44 million people worldwide, as of 2016. Alzheimer's is a terminal disease for which there is currently no known cure. It is most commonly found in people over 65 years old, although a less-common form called Familial Alzheimer's disease, or "early-onset Alzheimer's", also occurs, affecting about 1%—5% of the total of Alzheimer's sufferers.[1]
Typically, the disease begins many years before it is diagnosed. In its early stages, short-term memory loss is the most common symptom, which is often initially thought by the sufferer to be caused by other factors, such as aging or stress.[2] Later symptoms of the disease include confusion, anger, mood swings, language breakdown, long-term memory loss, and the general "withdrawal" of the sufferer as his or her senses decline.[2][3] Gradually the sufferer will lose minor, and then major bodily functions, until death finally occurs.[4] Survival after diagnosis has been estimated to be between 5 and 20 years.[5][6]
Symptoms
Common symptoms of dementia include:
A decline in memory Changes in thinking skills Poor judgment and reasoning Decreased focus and attention Decreased language ability Negative changes in behavior
Although the symptoms are common, they are typically experienced in unique ways.[7] "Stages" are commonly referred to by professionals to describe the progressive nature of Alzheimer's (typically "early", "mid" and "late onset") but the symptoms can cross over these "boundaries" for many sufferers.
Diagnosis
The symptoms of Alzheimer's disease are generally reported to a doctor or physician when memory-loss (or symptoms surrounding memory loss) begins to pose a serious concern. When Alzheimer's disease is suspected, diagnosis is typically confirmed by a behavioural assessment, and some form of cognitive test. Often this is followed by a brain scan, such as a PET scan.[8]
Criteria
There are three sets of criteria for the clinical diagnoses of the spectrum of Alzheimer's disease: the 2013 fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5); the National Institute on Aging-Alzheimer's Association (NIA-AA) definition as revised in 2011; and the International Working Group criteria as revised in 2010. Three periods, which can span decades, define the progression of Alzheimer's disease from the preclinical phase, to mild cognitive impairment (MCI), followed by Alzheimer's disease dementia.
Eight intellectual domains are most commonly impaired in AD: memory, language, perceptual skills, attention, motor skills, orientation, problem solving, and executive functional abilities, as listed in the fourth text revision of the DSM (DSM-IV-TR).
The DSM-5 defines criteria for probable or possible Alzheimer's for both major and mild neurocognitive disorder. Major or mild neurocognitive disorder must be present along with at least one cognitive deficit for a diagnosis of either probable or possible AD.
Laboratory tests
Apolipoprotein E4
Although apolipoprotein E4 is an important susceptibility gene for Alzheimer's disease][9], its sensitivity and specificity are insufficient (65 and 68 percent, respectively) to be used as a diagnostic test.[10]
However, the level of apolipoprotein E4 in cerebrospinal fluid may be predictive.[11]
Amyloid-beta protein
Amyloid-beta protein may be elevated in the cerebrospinal fluid of some patients.[11]
Autopsy
Completely reliable confirmation of a Alzheimer's diagnosis has traditionally only been possible upon the death of the patient. The brain tissue will be examined by a pathologist, and there are two unique lesions that are unmistakable signs of Alzheimer's that will give definite confirmation.
Cause/etiology
The etiology of Alzheimer's disease is incompletely understood. Alzheimer's disease is associated with senile plaques and neurofibrillary tangles in the brain.[12] The incorrect folding of proteins leads to the formation of amyloid plaques. The prion protein (PrPC) may be the cellular receptor for amyloid-beta oligomer.[13] Current research aims to determine if such plaques are the result of, or the cause of, Alzheimer's disease.
Regarding biomarkers, one study found that "a reduction in the cerebrospinal fluid (CSF) Aβ42 level denotes a pathophysiological process that significantly departs from normality (i.e., becomes dynamic) early, whereas the CSF total tau level and the adjusted hippocampal volume are biomarkers of downstream pathophysiological processes". [14]
Although there is a genetic susceptibility in a small percentage of people, Alzheimer's is not considered a genetic illness. The greatest risk factor for developing Alzheimer's is age, as the percentage of people developing it dramatically increases after age 65 and beyond. Major environmental/lifestyle risk factors include a diet high in saturated fats that are typical of the standard western diet, smoking, and long-term exposure to environmental toxins such as aluminium. Research is ongoing.
Alzheimer's appears to be a disease of the modern world, especially developed societies. Prior to the early 20th century, this dementia was completely unknown to science and was never described in elder members of traditional societies.
Individuals with the genetic disease Down syndrome (trisomy 21) are at much higher risk for developing Alzheimer's in their middle age. According to the National Down Syndrome Society, about 30% of people with Down syndrome who are in their 50s, and about 50% of those in their 60s, have Alzheimer’s disease. The lifetime risk is over 90%. [15]
Epidemiology
In the United States in 2020, Alzheimer's dementia prevalence was estimated to be 5% for those in the 60–74 age group, with the rate increasing to 14% in the 74–84 group and to 35% in those greater than 85.[16]
Advancing age is a primary risk factor for the disease and incidence rates are not equal for all ages: every 5 years after the age of 65, the risk of acquiring the disease approximately doubles, in developed societies such as Spain and Italy.
Treatment
As of 2024, no treatment has been found to stop progression or completely reverse the disease, but some treatments do slow the progression. Many preventive measures have been suggested for Alzheimer's disease, but their values are uncertain: mental stimulation, exercise, and a Mediterranean style diet are usually recommended, both as possible prevention and as a sensible way of managing the disease.[17]
Medications
As of 2023, available medications offer relatively small symptomatic benefit for some patients but generally do not slow disease progression. Newer monoclonal antibody treatments against beta-amyloid protein, for example, the drug Leqembi, have been approved in the United States, but they only slightly reduce the rate of disease progression and have significant side effects, including brain bleeding and in rare cases, death. These drugs cost over $20,000 a year, which could be prohibitively expensive for many patients.
Randomized controlled trials showed either small or absent benefit from acetylcholinesterase inhibitors[18] such as donepezil.[19][20][21]
The N-methyl-d-aspartate receptor antagonist memantine has shown some effectiveness[22] but does not add benefit to donepezil .[21]
Care management
Due to the incurable and degenerative nature of the disease care-management of Alzheimer's is essential. The role of the main caregiver is often taken by the spouse or a close relative.[23] Caregivers may themselves suffer from stress, over-work, depression, and from being physically assailed.[24]
Alzheimer's in society
Famous people who have, or have died of Alzheimer's disease, are the US president Ronald Reagan, the UK Prime minister Harold Wilson, the writers Terry Pratchett and Iris Murdoch, and the film stars Rita Hayworth and Charlton Heston.
History

Alzheimer’s disease is named after Dr. Alois Alzheimer, the German physician who first described it. In 1901, Alzheimer observed a 51-year-old patient at the Frankfurt asylum named Auguste Deter. Her symptoms included memory loss, language problems, and unpredictable behavior. In 1906, she died and Dr. Alzheimer noticed unusual pathology in her brain tissue; he found many abnormal clumps (now called amyloid plaques) and tangled bundles of fibers (now called neurofibrillary, or tau, tangles).
Prior to the early 20th century, Alzheimer's was undescribed in world medical literature. Other dementias and brain and behavioral disorders, however, were well documented by then.
References
- ↑ Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M (2005). "Global prevalence of dementia: a Delphi consensus study". Lancet 366 (9503): 2112–2117. DOI:10.1016/S0140-6736(05)67889-0. PMID 16360788. Research Blogging.
- ↑ 2.0 2.1 Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B (2007). "Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline". European Journal of Neurology 14 (1): E1–26. DOI:10.1111/j.1468-1331.2006.01605.x. PMID 17222085. Research Blogging.
- ↑ Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP (2005). "A 10-item smell identification scale related to risk for Alzheimer's disease". Ann. Neurol. 58 (1): 155–60. DOI:10.1002/ana.20533. PMID 15984022. Research Blogging.
- ↑ Understanding Stages and Symptoms of Alzheimer's Disease. National Institute on Aging (2007-10-26). Retrieved on 2008-02-21.
- ↑ Alzheimer's Disease Information Page. National Institute of Neurological Disorders and Stroke (NINDS) (2008-02-07). Retrieved on 2008-02-12.
- ↑ Alzheimer's Disease Treatment and Prognosis. Healthlink. Retrieved on 2008-02-15.
- ↑ What is Alzheimer’s disease?. www.alzheimers.org.uk (August 2007). Retrieved on 2008-02-21.
- ↑ Alzheimer's Diagnosis of AD. Alzheimer's Reearch Trust. Retrieved on 2008-02-29.
- ↑ Skoog I (August 2000). "Detection of preclinical Alzheimer's disease". N. Engl. J. Med. 343 (7): 502–3. PMID 10944568. “The APOE 4 allele is a susceptibility gene for Alzheimer's disease and seems to affect the age of onset of the disease. However, the presence of this allele alone is not sufficient to predict which asymptomatic subjects will ultimately have Alzheimer's disease, and the disease never develops in many subjects with this genotype” [e]
- ↑ Kivipelto M, Helkala EL, Laakso MP, et al (August 2002). "Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease". Ann. Intern. Med. 137 (3): 149–55. PMID 12160362. [e]
- ↑ 11.0 11.1 De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP et al. (2010). "Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people.". Arch Neurol 67 (8): 949-56. DOI:10.1001/archneurol.2010.179. PMID 20697045. Research Blogging.
- ↑ Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (2004). "The importance of neuritic plaques and tangles to the development and evolution of AD". Neurology 62 (11): 1984–1989. PMID 15184601. [e]
- ↑ Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (February 2009). "Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers". Nature 457 (7233): 1128–32. DOI:10.1038/nature07761. PMID 19242475. Research Blogging.
- ↑ Jack CR, Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ et al. (2011). "Evidence for ordering of Alzheimer disease biomarkers.". Arch Neurol 68 (12): 1526-35. DOI:10.1001/archneurol.2011.183. PMID 21825215. Research Blogging.
- ↑ National Down Syndrome Society. Alzheimer’s Disease and Down Syndrome. Available at: https://www.ndss.org/resources/ alzheimers/. Accessed July 11, 2024
- ↑ (May 2021) "Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020-2060)". Alzheimer's & Dementia 17 (12): 1966–1975. DOI:10.1002/alz.12362. PMID 34043283. PMC 9013315. Research Blogging.
- ↑ The Search for AD Prevention Strategies. National Institute on Aging (2006-08-29). Retrieved on 2008-02-29.
- ↑ Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H, van den Bussche H (2005). "Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials.". BMJ 331 (7512): 321-7. PMID 16081444.
- ↑ Howard RJ, Juszczak E, Ballard CG, et al (2007). "Donepezil for the treatment of agitation in Alzheimer's disease". N. Engl. J. Med. 357 (14): 1382–92. DOI:10.1056/NEJMoa066583. PMID 17914039. Research Blogging.
- ↑ Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, Lendon C, Shaw H, Bentham P (2004). "Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial.". Lancet 363 (9427): 2105-15. PMID 15220031.
- ↑ 21.0 21.1 Donepezil and Memantine for Moderate-to-Severe Alzheimer's Disease. NEJM 2012. http://dx.doi.org/10.1056/NEJMoa1106668
- ↑ Areosa Sastre A, McShane R, Sherriff F. "Memantine for dementia.". Cochrane Database Syst Rev: CD003154. PMID 15495043.
- ↑ O’Donovan ST. "Dementia caregiving burden and breakdown" (PDF). Retrieved on 2008-02-29.
- ↑ Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G (2007). "Systematic review of the effect of psychological interventions on family caregivers of people with dementia". Journal of Affective Disorders 101 (1-3): 75–89. DOI:10.1016/j.jad.2006.10.025. PMID 17173977. Research Blogging.