Interspike interval histogram: Difference between revisions
imported>Gareth Leng No edit summary |
imported>Daniel Mietchen m (intralinks) |
||
| (18 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
An '''interspike-interval histogram''' is one of the ways by which experimental neurophysiologists analyse the electrical behaviour of [[neuron]]s in the brain. | An '''interspike-interval histogram''' is one of the ways by which experimental neurophysiologists analyse the electrical behaviour of [[neuron]]s in the brain. | ||
One of the | One of the simplest ways to study the patterning of [[action potential|spike activity]] in a neuron is to record the spikes from a single neuron, using a fine [[microelectrode]], and then to construct an interspike interval histogram. This is simply a plot of the distribution of the observed [[interspike interval|times between spikes]] (t1, t2, t3, etc) collected in ‘bins’ of fixed width, say 1 ms. Typically, such a histogram would be constructed from about 2000 such intervals; if a neuron is firing at an average rate of about 5 spikes/s this will need a recording of 5-10 min of activity. | ||
[[Image:ISIH.png|thumb|300 px| Interspike interval distribution of the spontaneous activity of a typical magnocellular [[oxytocin]] neuron recorded from the rat [[supraoptic nucleus]]. The curve is a single negative exponential curve fitted to the tail of the histogram.]] | |||
The figure on the right shows the interspike interval histogram recorded from a single neuron in the [[hypothalamus]]. Immediately after a spike, a neuron has an absolute refractory period in which it is unable to fire another spike, so the first few bins of the histogram will be empty – the histogram starts from zero. After this, the neuron has a relative refractory period, during which it is hard, but not impossible, to trigger another spike, and as this relative [[refractory period]] gradually decays, the interspike interval distribution rises to its peak value – the mode of the histogram, in this example at about 50 ms. The tail of the histogram (from about 50 ms on) declines exponentially to zero, as shown by the fitted line. This exponentially declining tail is an indication that, after about 50 ms after a spike, spikes are triggered by an essentially random process. This follows because the distribution of inter-event times for a wholly random process fits a single negative exponential, as first observed by [[Simeon Denis Poisson|Poisson]]. | |||
In this example, the neuron has quite a prolonged period of relative inexcitability after each spike, and so we might predict that this cell is one that shows a long [[hyperpolarsising after-potential]] (HAP) after each spike. For this particular cell, this is wholly correct; the cell is an oxytocin cell, and in these cells spikes trigger a large influx of calcium into the cell, and (in these cells) this causes a prolonged opening of a class of [[calcium-activated potassium channel]]s, and this produces a relatively long HAP. | |||
==Examples in recent papers== | |||
* Witham CL, Baker SN (2007) Network oscillations and intrinsic spiking rhythmicity do not covary in monkey sensorimotor areas ''J Physiol'' [http://jp.physoc.org/cgi/content/full/580/3/801 508:801-13] | |||
* Zhang X, Carney LH (2005) Response properties of an integrate-and-fire model that receives subthreshold inputs ''Neural Comput''[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=16212763 17:2571–601] | |||
* Sabatier N ''et al.'' (2004) Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. ''J Physiol'' 558:161-80. PMID 15146047 | |||
Latest revision as of 05:58, 22 December 2010
An interspike-interval histogram is one of the ways by which experimental neurophysiologists analyse the electrical behaviour of neurons in the brain.
One of the simplest ways to study the patterning of spike activity in a neuron is to record the spikes from a single neuron, using a fine microelectrode, and then to construct an interspike interval histogram. This is simply a plot of the distribution of the observed times between spikes (t1, t2, t3, etc) collected in ‘bins’ of fixed width, say 1 ms. Typically, such a histogram would be constructed from about 2000 such intervals; if a neuron is firing at an average rate of about 5 spikes/s this will need a recording of 5-10 min of activity.
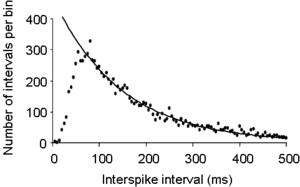
The figure on the right shows the interspike interval histogram recorded from a single neuron in the hypothalamus. Immediately after a spike, a neuron has an absolute refractory period in which it is unable to fire another spike, so the first few bins of the histogram will be empty – the histogram starts from zero. After this, the neuron has a relative refractory period, during which it is hard, but not impossible, to trigger another spike, and as this relative refractory period gradually decays, the interspike interval distribution rises to its peak value – the mode of the histogram, in this example at about 50 ms. The tail of the histogram (from about 50 ms on) declines exponentially to zero, as shown by the fitted line. This exponentially declining tail is an indication that, after about 50 ms after a spike, spikes are triggered by an essentially random process. This follows because the distribution of inter-event times for a wholly random process fits a single negative exponential, as first observed by Poisson.
In this example, the neuron has quite a prolonged period of relative inexcitability after each spike, and so we might predict that this cell is one that shows a long hyperpolarsising after-potential (HAP) after each spike. For this particular cell, this is wholly correct; the cell is an oxytocin cell, and in these cells spikes trigger a large influx of calcium into the cell, and (in these cells) this causes a prolonged opening of a class of calcium-activated potassium channels, and this produces a relatively long HAP.
Examples in recent papers
- Witham CL, Baker SN (2007) Network oscillations and intrinsic spiking rhythmicity do not covary in monkey sensorimotor areas J Physiol 508:801-13
- Zhang X, Carney LH (2005) Response properties of an integrate-and-fire model that receives subthreshold inputs Neural Comput17:2571–601
- Sabatier N et al. (2004) Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol 558:161-80. PMID 15146047