Imidacloprid: Difference between revisions
imported>Gerald Zuckier (reffed) |
mNo edit summary |
||
| (10 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Imidacloprid.jpg|center|thumb|200px]] | |||
|width=200px | |||
|molname=imidacloprid | |||
|synonyms= | |||
|molformula= C<sub>9</sub>H<sub>10</sub>ClN<sub>5</sub>O<sub>2</sub> | |||
|molmass= | |||
|uses=insecticide | |||
|properties=nicotine analog | |||
|hazards=see below | |||
|iupac= see chemistry | |||
|casnumber= | |||
}} | |||
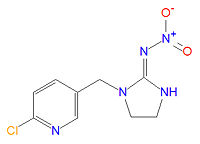
'''Imidacloprid''' ([[IUPAC]] name (EZ)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine) is an [[insecticide]] manufactured by [[Bayer Cropscience]] (part of the drug and chemical conglomerate [[Bayer|Bayer AG]]). It is or has been sold in various formulations, alone or mixed with other active ingredients, in over 100 countries under the [[trade name]]s Admire, Advantage, Aeris, Akteur, Amigo, Amistar Admire, Amparo, Ancla, Audax, Baytan Secur, Beam Admire, Bilogic, Blindage, Carrena, Cereline Secur, Chinook, Confidor, Confidor Supra, Confidor Ultra, Conidan, Conidor, CropStar, El Hombre, Escocet, Faibel, Ferialo Bl, Ferial Blé, Férial Orge, Gaucho, Gaucho Bl, Gaucho Blé, Gaucho CS, Gaucho Grande, Gaucho M, Gaucho Maícero, Gaucho MT, Gaucho MZ, Gaucho Orge, Gaucho Primo, Gaucho Rx 246 FS, Gaucho T, Gaucho XT, Genesis, Hachikusan, Imprimo, Leverage, Manta Plus, Merit, Monceren Extra, Monceren G, Monceren GT, Monceren Admire, Monceren Star, Montur, Poncho, Premier, Premise, Prestige, Prestige M, Provado, Raxil Secur, Seed-one, Sibutol Secur, Taifun, Traffic, Win Admire, Winner, Yunta, and Zorro FS 236,3. | '''Imidacloprid''' ([[IUPAC]] name (EZ)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine) is an [[insecticide]] manufactured by [[Bayer Cropscience]] (part of the drug and chemical conglomerate [[Bayer|Bayer AG]]). It is or has been sold in various formulations, alone or mixed with other active ingredients, in over 100 countries under the [[trade name]]s Admire, Advantage, Aeris, Akteur, Amigo, Amistar Admire, Amparo, Ancla, Audax, Baytan Secur, Beam Admire, Bilogic, Blindage, Carrena, Cereline Secur, Chinook, Confidor, Confidor Supra, Confidor Ultra, Conidan, Conidor, CropStar, El Hombre, Escocet, Faibel, Ferialo Bl, Ferial Blé, Férial Orge, Gaucho, Gaucho Bl, Gaucho Blé, Gaucho CS, Gaucho Grande, Gaucho M, Gaucho Maícero, Gaucho MT, Gaucho MZ, Gaucho Orge, Gaucho Primo, Gaucho Rx 246 FS, Gaucho T, Gaucho XT, Genesis, Hachikusan, Imprimo, Leverage, Manta Plus, Merit, Monceren Extra, Monceren G, Monceren GT, Monceren Admire, Monceren Star, Montur, Poncho, Premier, Premise, Prestige, Prestige M, Provado, Raxil Secur, Seed-one, Sibutol Secur, Taifun, Traffic, Win Admire, Winner, Yunta, and Zorro FS 236,3. | ||
<ref name = "chin">[http://www.agrochemchina.com/products_1_6.asp Imidacloprid], [[Agrochemchina]] product page.</ref>, <ref name="bayer"> [http://www.agrocourier.com/bayer/cropscience/cscms.nsf/id/imidacloprid_se.htm?Open Imidacloprid], [[Bayer Cropscience]] product page.</ref>, <ref>[http://www.bayercropscience.com.ar/arg/pages/page.php?id=InfoConnect Connect], Bayer Cropscience Argentina product page.</ref>, <ref>[http://www.cda.sp.gov.br/CFICS30082006%20-%20PRODUTOS.pdf Produtos cadastrados], Secretaria de Agricultura e Abastecimento, Coordenadoria de Defesa Agropecuária, Centro de Fiscalização de Insumos e Conservação do Solo.</ref> Uses include agriculturally as a seed dressing, soil treatment or foliar treatment for [[crop]]s and [[turf]], as a termite preventive, and as a [[flea]] treatment for [[pet]]s.<ref name = "chin"/> | <ref name = "chin">[http://www.agrochemchina.com/products_1_6.asp Imidacloprid], [[Agrochemchina]] product page.</ref>, <ref name="bayer"> [http://www.agrocourier.com/bayer/cropscience/cscms.nsf/id/imidacloprid_se.htm?Open Imidacloprid], [[Bayer Cropscience]] product page.</ref>, <ref>[http://www.bayercropscience.com.ar/arg/pages/page.php?id=InfoConnect Connect], Bayer Cropscience Argentina product page.</ref>, <ref>[http://www.cda.sp.gov.br/CFICS30082006%20-%20PRODUTOS.pdf Produtos cadastrados], Secretaria de Agricultura e Abastecimento, Coordenadoria de Defesa Agropecuária, Centro de Fiscalização de Insumos e Conservação do Solo.</ref> Uses include agriculturally as a seed dressing, soil treatment or foliar treatment for [[crop]]s and [[turf]], as a [[termite]] preventive, and as a [[flea]] treatment for [[pet]]s.<ref name = "chin"/> | ||
Imidacloprid was first patented in the United States in U.S. Pat. No. 4,742,060, on | Imidacloprid was first patented in the United States in U.S. Pat. No. 4,742,060, on May 3, 1988, by Nihon Tokushu Noyaku Seizo K.K. of Tokyo, Japan. | ||
==Biochemistry== | ==Biochemistry== | ||
| Line 9: | Line 26: | ||
Imidacloprid is rated as "moderately toxic" acutely by the [[WHO]] and the [[EPA]] (class II or III, requiring a "Warning" or "Caution" label), and a "potential" [[ground water]] contaminant. It is rated as an "unlikely" [[carcinogen]] by the EPA (group E), and is not listed for [[endocrine]], [[reproductive]], or [[developmental]] toxicity, or as a chemical of special concern by any agencies. It is not banned, restricted, cancelled, or illegal to import in any countries. Tolerances for imidacloprid residue in food range from 0.02 ppm in eggs to 3.0 ppm in [[hops]]. | Imidacloprid is rated as "moderately toxic" acutely by the [[WHO]] and the [[EPA]] (class II or III, requiring a "Warning" or "Caution" label), and a "potential" [[ground water]] contaminant. It is rated as an "unlikely" [[carcinogen]] by the EPA (group E), and is not listed for [[endocrine]], [[reproductive]], or [[developmental]] toxicity, or as a chemical of special concern by any agencies. It is not banned, restricted, cancelled, or illegal to import in any countries. Tolerances for imidacloprid residue in food range from 0.02 ppm in eggs to 3.0 ppm in [[hops]]. | ||
Animal toxicity is similar to that of the parent compound, nicotine; [[fatigue]], [[twitch]]ing, [[cramp]]s, and [[weakness]] leading to [[asphyxia]]. The oral [[LD50]] (the dose which resulted in mortality of half of the test animals) of imidacloprid is 450 mg/kg body weight in [[rat]]s and 131 mg/kg in [[mouse|mice]]; the 24-hour [[dermal]] LD50 in rats is greater than 5,000 mg/kg. It is not irritating to eyes or skin in [[rabbit]]s and [[guinea pig]]s (although some commercial preparations contain [[clay]] as an inert ingredient, which may be an [[irritant]]). The acute [[inhalation]] LD50 in rats was not reached at the greatest attainable concentrations, 69 mg/cubic meter of air as an aerosol, and 5,323 mg/cubic meter of air as a dust. In rats subjected to a two year feeding study, no observable effect was seen at 100 ppm. At 300 ppm females showed decreased body weight gain and males showed increased [[thyroid]] [[lesion]]s, while females showed increased thyroid lesions at 900 ppm. In a one year feeding study in dogs, no observable effect was seen at 1,250 ppm, while levels up to 2,500 ppm led to [[hypercholesterolemia]] and elevated [[liver]] [[Cytochrome_P-450#Drug_Metabolism|cytochrome p-450 measurements]]. Reproductive studies in rats resulted in no observable effect at 100 ppm and decreased pup weight at 250 ppm; developmental toxicity studies in rats showed no observable effect at 30 mg/kg/day and skeletal anomalies at 100 mg/kg/day, while in rabbits no observable effect was detected at 24 mg/kg/day and skeletal abnormalities at 72 mg/kg/day. Imidacloprid was negative for [[mutagenicity]] in 21 out of 23 different laboratory tests, but was positive for [[chromosome|chromosomal]] changes in human [[lymphocyte]]s and for [[genotoxic]]ity in [[CHO cell]]s. No [[carcinogenicity]] was seen in rats fed up to 1,800 ppm of imidacloprid for two years. <ref> [http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/imidacloprid-ext.html Cornell Extension Toxicology Network]</ref> | Animal toxicity is similar to that of the parent compound, nicotine; [[fatigue]], [[twitch]]ing, [[cramp]]s, and [[weakness]] leading to [[asphyxia]]. The oral [[LD50]] (the dose which resulted in mortality of half of the test animals) of imidacloprid is 450 mg/kg body weight in [[rat]]s and 131 mg/kg in [[mouse|mice]]; the 24-hour [[dermal]] LD50 in rats is greater than 5,000 mg/kg. It is not irritating to eyes or skin in [[rabbit]]s and [[guinea pig]]s (although some commercial preparations contain [[clay]] as an inert ingredient, which may be an [[irritant]]). The acute [[inhalation]] LD50 in rats was not reached at the greatest attainable concentrations, 69 mg/cubic meter of air as an aerosol, and 5,323 mg/cubic meter of air as a dust. In rats subjected to a two year feeding study, no observable effect was seen at 100 ppm. At 300 ppm females showed decreased body weight gain and males showed increased [[thyroid]] [[lesion]]s, while females showed increased thyroid lesions at 900 ppm. In a one year feeding study in dogs, no observable effect was seen at 1,250 ppm, while levels up to 2,500 ppm led to [[hypercholesterolemia]] and elevated [[liver]] [[Cytochrome_P-450#Drug_Metabolism|cytochrome p-450 measurements]]. Reproductive studies in rats resulted in no observable effect at 100 ppm and decreased pup weight at 250 ppm; developmental toxicity studies in rats showed no observable effect at 30 mg/kg/day and skeletal anomalies at 100 mg/kg/day, while in rabbits no observable effect was detected at 24 mg/kg/day and skeletal abnormalities at 72 mg/kg/day. Imidacloprid was negative for [[mutagenicity]] in 21 out of 23 different laboratory tests, but was positive for [[chromosome|chromosomal]] changes in human [[lymphocyte]]s and for [[genotoxic]]ity in [[CHO cell]]s. No [[carcinogenicity]] was seen in rats fed up to 1,800 ppm of imidacloprid for two years. <ref> [http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/imidacloprid-ext.html Cornell Extension Toxicology Network]</ref>,<ref name="chin"/> | ||
A desirable property of imidacloprid is its low [[vapor pressure]], which makes unwanted airborne contamination impossible. The chemical breaks down completely to [[inorganic]] molecules by both [[photolysis]] and [[microbe|microbial]] action, extremely rapidly in the air and with a [[half-life]] of 30 days in water and 27 days in [[soil]] [[anaerobic]]ally. Although it is not "persistent" in the technical sense since it does degrade, it can, however, have a half-life in soil under [[aerobic]] conditions of as long as 997 days, which is the cause of the concern over possible water contamination as it gradually leaches out of a hypothetical soil reservoir. The manufacturer maintains that, when applied according to instructions, such long-term contamination is only found as the result of "repetitive application over several years" and spread to beneficial insect populations is minimal. In the body, 96% of the chemical is eliminated within 48 hours; the most important degradation product in the body is 6-chloronicotinic acid, another nicotinic neurotoxin with similar properties. Imidacloprid has, however, been reported to degrade into toxic, persistent, 2-chloro[[pyridine]]. | A desirable property of imidacloprid is its low [[vapor pressure]], which makes unwanted airborne contamination impossible. The chemical breaks down completely to [[inorganic]] molecules by both [[photolysis]] and [[microbe|microbial]] action, extremely rapidly in the air and with a [[half-life]] of 30 days in water and 27 days in [[soil]] [[anaerobic]]ally. Although it is not "persistent" in the technical sense since it does degrade, it can, however, have a half-life in soil under [[aerobic]] conditions of as long as 997 days, which is the cause of the concern over possible water contamination as it gradually leaches out of a hypothetical soil reservoir. The manufacturer maintains that, when applied according to instructions, such long-term contamination is only found as the result of "repetitive application over several years" and spread to beneficial insect populations is minimal. In the body, 96% of the chemical is eliminated within 48 hours; the most important degradation product in the body is 6-chloronicotinic acid, another nicotinic neurotoxin with similar properties. Imidacloprid has, however, been reported to degrade into toxic, persistent, 2-chloro[[pyridine]]. | ||
==Agricultural uses== | ==Agricultural uses== | ||
The most widely used agricultural applications for imidacloprid in [[California]] are pest control in structures, [[turf]] pest control, [[grape]] growing, and head and leaf [[lettuce]] growing. Other widespread crop uses are [[rice]], [[cereal]], [[maize]], [[potato]]es, [[vegetable]]s, [[sugar beet]]s, [[pome fruit]], [[cotton]], [[canola]] or [[rapeseed]], [[stone fruit]], [[pasture]], [[citrus]], [[sorghum]], and [[sunflower]]s.<ref name="bayer"/>,<ref name="chin"/> Target insects include sucking insects (''e.g.'' [[aphid]]s, [[whitefly|whiteflies]], [[leafhopper]]s and [[planthopper]]s, [[thrip]]s, [[scale]]s, [[mealybug]]s, [[Hemiptera|bugs]], [[psyllid]]s, and [[phylloxera]]), [[beetle]]s (''e.g.'' [[leaf beetle]]s, [[Colorado potato beetle]]s, [[weevil]]s, [[wireworm]]s, [[grub]]s, [[pygmy beetle]]s, and [[flea beetle]]s), and others (''e.g.'' [[lepidoptera|lepidopterous]] [[leafminer]]s, some [[diptera]] such as [[fruit fly|fruit flies]], [[termite]]s, [[locust]]s, and [[flea]]s).<ref name="bayer"/>,<ref name="chin"/> | The most widely used agricultural applications for imidacloprid in [[California (U.S. state)]] are pest control in structures, [[turf]] pest control, [[grape]] growing, and head and leaf [[lettuce]] growing. Other widespread crop uses are [[rice]], [[cereal]], [[maize]], [[potato]]es, [[vegetable]]s, [[sugar beet]]s, [[pome fruit]], [[cotton]], [[canola]] or [[rapeseed]], [[stone fruit]], [[pasture]], [[citrus]], [[sorghum]], and [[sunflower]]s.<ref name="bayer"/>,<ref name="chin"/> Target insects include sucking insects (''e.g.'' [[aphid]]s, [[whitefly|whiteflies]], [[leafhopper]]s and [[planthopper]]s, [[thrip]]s, [[scale (insect)|scale]]s, [[mealybug]]s, [[Hemiptera|bugs]], [[psyllid]]s, and [[phylloxera]]), [[beetle]]s (''e.g.'' [[leaf beetle]]s, [[Colorado potato beetle]]s, [[weevil]]s, [[wireworm]]s, [[grub]]s, [[pygmy beetle]]s, and [[flea beetle]]s), and others (''e.g.'' [[lepidoptera|lepidopterous]] [[leafminer]]s, some [[diptera]] such as [[fruit fly|fruit flies]], [[termite]]s, [[locust]]s, and [[flea]]s).<ref name="bayer"/>,<ref name="chin"/> | ||
===A systemic insecticide=== | ===A systemic insecticide=== | ||
| Line 20: | Line 37: | ||
==Controversy== | ==Controversy== | ||
In [[France]], imidacloprid (as Gaucho) became controversial in terms of a possible link to derangement of behavior in domesticated [[honeybee]]s; the manufacturer's position is that bees would not be exposed to the compound if it is applied as directed. Since then, imacloprid has received increased attention as a possible factor in [[Colony Collapse Disorder]], a mysterious condition that causes sudden death of [[honey bee]] populations, although no link has been demonstrated. | In [[France]], imidacloprid (as Gaucho) became controversial in terms of a possible link to derangement of behavior in domesticated [[honeybee]]s; the manufacturer's position is that bees would not be exposed to the compound if it is applied as directed.<ref>[http://www.honeycouncil.ca/users/folder.asp?FolderID=4969 Gaucho / Admire] [[Canadian Honey Council]].</ref> Since then, imacloprid has received increased attention as a possible factor in [[Colony Collapse Disorder]], a mysterious condition that causes sudden death of [[honey bee]] populations, although no link has been demonstrated.<ref>[http://www.honeycouncil.ca/users/folder.asp?FolderID=4983 Gaucho / Admire: Research in Canada] [[Canadian Honey Council]].</ref> | ||
==References== | ==References== | ||
| Line 29: | Line 46: | ||
==External links== | ==External links== | ||
*[http://arjournals.annualreviews.org/doi/full/10.1146/annurev.pharmtox.45.120403.095930 NEONICOTINOID INSECTICIDE TOXICOLOGY: Mechanisms of Selective Action, Ann. Rev. Pharm. and Tox. 45, 247-68 (subscribers only)] | *[http://arjournals.annualreviews.org/doi/full/10.1146/annurev.pharmtox.45.120403.095930 NEONICOTINOID INSECTICIDE TOXICOLOGY: Mechanisms of Selective Action, Ann. Rev. Pharm. and Tox. 45, 247-68 (subscribers only)] | ||
*[http://www.flora.org/healthyottawa/merit-pesticide-insecticide-grub.htm Breakdown Chart of Imidacloprid forming toxic 2-chloro pyridine] | *[http://www.flora.org/healthyottawa/merit-pesticide-insecticide-grub.htm Breakdown Chart of Imidacloprid forming toxic 2-chloro pyridine][[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:00, 31 August 2024
|
| |||||||
| imidacloprid | |||||||
| |||||||
| Uses: | insecticide | ||||||
| Properties: | nicotine analog | ||||||
| Hazards: | see below | ||||||
| |||||||
Imidacloprid (IUPAC name (EZ)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine) is an insecticide manufactured by Bayer Cropscience (part of the drug and chemical conglomerate Bayer AG). It is or has been sold in various formulations, alone or mixed with other active ingredients, in over 100 countries under the trade names Admire, Advantage, Aeris, Akteur, Amigo, Amistar Admire, Amparo, Ancla, Audax, Baytan Secur, Beam Admire, Bilogic, Blindage, Carrena, Cereline Secur, Chinook, Confidor, Confidor Supra, Confidor Ultra, Conidan, Conidor, CropStar, El Hombre, Escocet, Faibel, Ferialo Bl, Ferial Blé, Férial Orge, Gaucho, Gaucho Bl, Gaucho Blé, Gaucho CS, Gaucho Grande, Gaucho M, Gaucho Maícero, Gaucho MT, Gaucho MZ, Gaucho Orge, Gaucho Primo, Gaucho Rx 246 FS, Gaucho T, Gaucho XT, Genesis, Hachikusan, Imprimo, Leverage, Manta Plus, Merit, Monceren Extra, Monceren G, Monceren GT, Monceren Admire, Monceren Star, Montur, Poncho, Premier, Premise, Prestige, Prestige M, Provado, Raxil Secur, Seed-one, Sibutol Secur, Taifun, Traffic, Win Admire, Winner, Yunta, and Zorro FS 236,3. [1], [2], [3], [4] Uses include agriculturally as a seed dressing, soil treatment or foliar treatment for crops and turf, as a termite preventive, and as a flea treatment for pets.[1]
Imidacloprid was first patented in the United States in U.S. Pat. No. 4,742,060, on May 3, 1988, by Nihon Tokushu Noyaku Seizo K.K. of Tokyo, Japan.
Biochemistry
A chlorinated analog of nicotine, the compound therefore belongs to the class of chloronicotinyl insecticides, and acts on the nicotinic acetylcholine receptor; the chlorination inhibits degradation by acetylcholine-esterase. Imidacloprid is notable for its relatively low toxicity to most animals other than insects due to its specificity for this type of receptor, which is found more often in insect nervous systems and zooplankton than that of other animals (exceptions exist; earthworms and a few species of fish, for example). This improved ratio of toxicity allows the use of very low concentrations (e.g. 0.05-0.125 lbs/acre), making it safer for insect control than other neurotoxins (particularly organophosphates) and enabling its use in applications as diverse as flea treatments for pets, control of beetle larvae in lawns, eradication or prevention of termite infestation in buildings, and other uses where animals and people may be exposed. Imidacloprid is, for example, present as a main (or the sole) active ingredient in concentrations between five and ten percent in three out of the four most widely used flea treatment and preventative topical treatments for dogs in the United States; these manufacturers claim an effective killing persistence of at least four weeks. The compound is also used for flea treatment on cats, whose livers have only limited detoxification ability compared to dogs and humans.
Imidacloprid is rated as "moderately toxic" acutely by the WHO and the EPA (class II or III, requiring a "Warning" or "Caution" label), and a "potential" ground water contaminant. It is rated as an "unlikely" carcinogen by the EPA (group E), and is not listed for endocrine, reproductive, or developmental toxicity, or as a chemical of special concern by any agencies. It is not banned, restricted, cancelled, or illegal to import in any countries. Tolerances for imidacloprid residue in food range from 0.02 ppm in eggs to 3.0 ppm in hops.
Animal toxicity is similar to that of the parent compound, nicotine; fatigue, twitching, cramps, and weakness leading to asphyxia. The oral LD50 (the dose which resulted in mortality of half of the test animals) of imidacloprid is 450 mg/kg body weight in rats and 131 mg/kg in mice; the 24-hour dermal LD50 in rats is greater than 5,000 mg/kg. It is not irritating to eyes or skin in rabbits and guinea pigs (although some commercial preparations contain clay as an inert ingredient, which may be an irritant). The acute inhalation LD50 in rats was not reached at the greatest attainable concentrations, 69 mg/cubic meter of air as an aerosol, and 5,323 mg/cubic meter of air as a dust. In rats subjected to a two year feeding study, no observable effect was seen at 100 ppm. At 300 ppm females showed decreased body weight gain and males showed increased thyroid lesions, while females showed increased thyroid lesions at 900 ppm. In a one year feeding study in dogs, no observable effect was seen at 1,250 ppm, while levels up to 2,500 ppm led to hypercholesterolemia and elevated liver cytochrome p-450 measurements. Reproductive studies in rats resulted in no observable effect at 100 ppm and decreased pup weight at 250 ppm; developmental toxicity studies in rats showed no observable effect at 30 mg/kg/day and skeletal anomalies at 100 mg/kg/day, while in rabbits no observable effect was detected at 24 mg/kg/day and skeletal abnormalities at 72 mg/kg/day. Imidacloprid was negative for mutagenicity in 21 out of 23 different laboratory tests, but was positive for chromosomal changes in human lymphocytes and for genotoxicity in CHO cells. No carcinogenicity was seen in rats fed up to 1,800 ppm of imidacloprid for two years. [5],[1]
A desirable property of imidacloprid is its low vapor pressure, which makes unwanted airborne contamination impossible. The chemical breaks down completely to inorganic molecules by both photolysis and microbial action, extremely rapidly in the air and with a half-life of 30 days in water and 27 days in soil anaerobically. Although it is not "persistent" in the technical sense since it does degrade, it can, however, have a half-life in soil under aerobic conditions of as long as 997 days, which is the cause of the concern over possible water contamination as it gradually leaches out of a hypothetical soil reservoir. The manufacturer maintains that, when applied according to instructions, such long-term contamination is only found as the result of "repetitive application over several years" and spread to beneficial insect populations is minimal. In the body, 96% of the chemical is eliminated within 48 hours; the most important degradation product in the body is 6-chloronicotinic acid, another nicotinic neurotoxin with similar properties. Imidacloprid has, however, been reported to degrade into toxic, persistent, 2-chloropyridine.
Agricultural uses
The most widely used agricultural applications for imidacloprid in California (U.S. state) are pest control in structures, turf pest control, grape growing, and head and leaf lettuce growing. Other widespread crop uses are rice, cereal, maize, potatoes, vegetables, sugar beets, pome fruit, cotton, canola or rapeseed, stone fruit, pasture, citrus, sorghum, and sunflowers.[2],[1] Target insects include sucking insects (e.g. aphids, whiteflies, leafhoppers and planthoppers, thrips, scales, mealybugs, bugs, psyllids, and phylloxera), beetles (e.g. leaf beetles, Colorado potato beetles, weevils, wireworms, grubs, pygmy beetles, and flea beetles), and others (e.g. lepidopterous leafminers, some diptera such as fruit flies, termites, locusts, and fleas).[2],[1]
A systemic insecticide
Imidacloprid is taken up by plant roots and diffuses in the plant vascular system, where insects ingest it by sucking the plant fluids. The products Confidor and Admire are meant for application via irrigation, application to the soil, or on foliage, while Gaucho is intended for use as a seed dressing, applied to the seed before sowing.
Controversy
In France, imidacloprid (as Gaucho) became controversial in terms of a possible link to derangement of behavior in domesticated honeybees; the manufacturer's position is that bees would not be exposed to the compound if it is applied as directed.[6] Since then, imacloprid has received increased attention as a possible factor in Colony Collapse Disorder, a mysterious condition that causes sudden death of honey bee populations, although no link has been demonstrated.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Imidacloprid, Agrochemchina product page.
- ↑ 2.0 2.1 2.2 Imidacloprid, Bayer Cropscience product page.
- ↑ Connect, Bayer Cropscience Argentina product page.
- ↑ Produtos cadastrados, Secretaria de Agricultura e Abastecimento, Coordenadoria de Defesa Agropecuária, Centro de Fiscalização de Insumos e Conservação do Solo.
- ↑ Cornell Extension Toxicology Network
- ↑ Gaucho / Admire Canadian Honey Council.
- ↑ Gaucho / Admire: Research in Canada Canadian Honey Council.