Leidenfrost effect: Difference between revisions
imported>Meg Taylor (update) |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 9: | Line 9: | ||
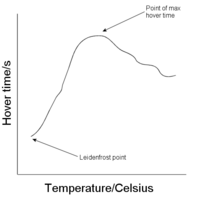
The Leidenfrost point varies for different liquids. It may be seen to rise as the carbon chain length increases in [[Homologous series|organic homologous series]]. For water it is about 230°C. It may also be observed how the time for which a droplet hovers (of a certain size) before evaporating completely for different liquids differ. The temperature for which this time is a maximum follows the same trend as the Leidenfrost point for a homologous series. | The Leidenfrost point varies for different liquids. It may be seen to rise as the carbon chain length increases in [[Homologous series|organic homologous series]]. For water it is about 230°C. It may also be observed how the time for which a droplet hovers (of a certain size) before evaporating completely for different liquids differ. The temperature for which this time is a maximum follows the same trend as the Leidenfrost point for a homologous series. | ||
[[Image:Leidenfrost-water-on- | [[Image:Leidenfrost-water-on-aluminium.jpg|thumb|200px|Image of a drop of water hovering on molten aluminium]][[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 11 September 2024
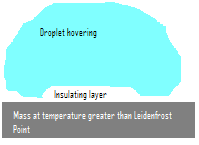
The Leidenfrost effect was first noted by Johann Gottlob Leidenfrost in 1756 in A Tract About Some Properties of Common Water. The phenomenon occurs when a liquid drop comes into contact with a mass which is hotter than the Leidenfrost point (which is significantly greater than the boiling point) of the liquid. When the liquid drop makes contact with the mass, a thin insulating layer of vapour forms between the hot object and the liquid drop which dramatically slows the evaporation of the remainder of the droplet, and the drop thus appears to hover over the plate as it slowly becomes smaller and smaller. The effect occurs due to the smaller conduction of heat through a vapour than through liquid.
The effect may be demonstrated at home by heating up a frying pan. When drops of oil or water are sprinkled onto the pan's surface they skitter across it as small balls, but only when the pan is hot enough - this is the Leidenfrost effect in action. It is also responsible for one being able to put a wet hand into molten lead without burning it or take a mouthful of liquid nitrogen (without breathing it in, of course).
The Leidenfrost point varies for different liquids. It may be seen to rise as the carbon chain length increases in organic homologous series. For water it is about 230°C. It may also be observed how the time for which a droplet hovers (of a certain size) before evaporating completely for different liquids differ. The temperature for which this time is a maximum follows the same trend as the Leidenfrost point for a homologous series.