Water: Difference between revisions
imported>Anthony.Sebastian (→The biology perspective: start text) |
mNo edit summary |
||
| (40 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC|right}} | {{TOC|right}} | ||
'''Water''' is a [[chemical compound]] composed of [[hydrogen]] and [[oxygen]] and has the [[chemical formula]] (H<sub>2</sub>O or HOH). At typical room [[temperature]] and [[pressure]], it is a [[liquid]] that is nearly colorless, tasteless, and odorless. Its [[molecular structure]] and other properties are discussed in one of the sections below. | '''Water''' is a [[chemical compound]] composed of [[hydrogen]] and [[oxygen]] and has the [[chemical formula]] (H<sub>2</sub>O or HOH). At typical room [[temperature]] and [[pressure]], it is a [[liquid]] that is nearly colorless, tasteless, and odorless. Its [[molecular structure]] and other properties are discussed in one of the sections below. | ||
| Line 10: | Line 9: | ||
==The earth sciences perspective== | ==The earth sciences perspective== | ||
{|align="right" border="0" width="375" | {|align="right" border="0" width="375" style="clear: both" | ||
| | | | ||
|{{Image|Earth Water Distribution.jpg|right|350px}} | |{{Image|Earth Water Distribution.jpg|right|350px}} | ||
| Line 52: | Line 51: | ||
| '''[[Melting point]]''' || 273.15 K (0 °C) | | '''[[Melting point]]''' || 273.15 K (0 °C) | ||
|- align="left" | |- align="left" | ||
|'''[[Freezing point]]''' || [[Water/Freezing point|{{:Water/Freezing point}}]] | |'''[[Freezing point]]''' || [[Water/Freezing point|{{:Water/Freezing point}}]]<ref>For more information on why the freezing point of pure water is not measurable see:[http://www.iapws.org/relguide/Ice-Rev2009.pdf Revised Release on the Equation of State 2006 for H2O Ice Ih ] The International Association for the Properties of Water and Steam, [[ The Netherlands]], September 2009</ref><ref>For more information on the [[Colligative properties|colligative property]] of freezing point depression of water by adding of a solvent (such as a salt) see:[http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/meltpt.html Freezing Point Depression in Solutions] Rod Nave, Department of Physics and Astronomy, [[Georgia State University]]</ref> | ||
|- align="left" | |- align="left" | ||
| '''[[Specific heat]], c<sub>p</sub>''' || 4.184 J/(g·K) for liquid at 20 °C | | '''[[Specific heat]], c<sub>p</sub>''' || 4.184 J/(g·K) for liquid at 20 °C | ||
| Line 66: | Line 65: | ||
|} | |} | ||
On a molecular level, water is a [[chemical compound]], a '[[molecule]]' comprised of two [[Atom|atoms]] of [[hydrogen]] bonded to one atom of [[oxygen]] (H<sub>2</sub>O) (see the two figures | On a molecular level, water is a [[chemical compound]], a '[[molecule]]' comprised of two [[Atom|atoms]] of [[hydrogen]] bonded to one atom of [[oxygen]] (H<sub>2</sub>O) (see the two figures below]. The two types of atoms bond together into a single structure by sharing the unpaired electrons in the outermost electron shells surrounding their nuclei — i.e., by 'covalent' bonds. We can give the least technical explanation of the phenomenon in the following way. Atoms are electrically neutral, having equal numbers of protons (charged positively, situated in the central nucleus) and electrons (charged negatively, surrounding the nucleus in one or more 'shells'). A hydrogen atom has a nucleus with one proton and one (unpaired) electron in its only electron shell. An oxygen has a nucleus with eight protons, with its counterbalancing eight electrons distributed two in its first shell and six in its next and outermost shell — of the latter, two remaining unpaired. For reasons requiring considerations of quantum mechanics, a hydrogen atom behaves as if it 'needed' one more electron to pair up with its sole electron in its first (and only) electron shell, to fill that shell to its natural capacity. An oxygen atom has its first electron shell filled to capacity with two electrons, but behaves as if it 'needed' two additional electrons to pair up with the two unpaired electrons among the six in its next (and outermost) shell, which has a natural capacity of eight electrons. Two hydrogen atoms can thus share electrons with one oxygen atom, filling their respective outermost electron shells to capacity, achieving a more stable state in the covalent bonds. | ||
{{Image| | |||
{{Image| | {{Image|Water molecule dimensions.png|left|150px|Schematic representation of a water molecule showing the relative positions of the constituent atoms.}} | ||
{{Image|Water molecules lg nsf gov.jpg|left|350px|Three-dimensional model of individual water molecules associating with each other in three different structural configurations. Oxygen atoms shown in red, hydrogen atoms in grey.}} | |||
The accompanying figures model the fact that the two hydrogen atoms do not bond symmetrically about the oxygen atom, but huddle somewhat closer together (~105<sup>o</sup>) than they would situated oppositely (180<sup>o</sup>) about the oxygen atom — the explanation of which requires knowledge of '[[Molecular orbital theory|molecular orbital theory]]'. The plane of the molecule forms an isosceles triangle. Because the total charge of the nucleus of the larger oxygen atom exceeds that of the two smaller hydrogen atoms, the electrons spend more time nearer the oxygen atom, rendering the charge distribution on the molecule asymmetrical, with more negativity at the oxygen 'pole' of the ''molecule'', and more positivity at the hydrogen 'pole' of the ''molecule''. Chemists refer to the water molecule as 'polar', something roughly akin to the magnetic polar nature of a bar magnet. They also refer to the two covalent bonds in the molecule as [http://academic.brooklyn.cuny.edu/biology/bio4fv/page/polar_c.htm 'polar covalent bonds']. | The accompanying figures model the fact that the two hydrogen atoms do not bond symmetrically about the oxygen atom, but huddle somewhat closer together (~105<sup>o</sup>) than they would situated oppositely (180<sup>o</sup>) about the oxygen atom — the explanation of which requires knowledge of '[[Molecular orbital theory|molecular orbital theory]]'. The plane of the molecule forms an isosceles triangle. Because the total charge of the nucleus of the larger oxygen atom exceeds that of the two smaller hydrogen atoms, the electrons spend more time nearer the oxygen atom, rendering the charge distribution on the molecule asymmetrical, with more negativity at the oxygen 'pole' of the ''molecule'', and more positivity at the hydrogen 'pole' of the ''molecule''. Chemists refer to the water molecule as 'polar', something roughly akin to the magnetic polar nature of a bar magnet. They also refer to the two covalent bonds in the molecule as [http://academic.brooklyn.cuny.edu/biology/bio4fv/page/polar_c.htm 'polar covalent bonds']. | ||
| Line 78: | Line 80: | ||
When cooled down to 0 K (absolute zero) or as close as can be practically achieved water is the only known substance (as of this date) that has a rest vibrational energy. No other known substance exhibits this peculiar behavior as it was assumed that at the absolute zero temperature every molecule would be totally frozen, having an energy of 0 J/mol. | When cooled down to 0 K (absolute zero) or as close as can be practically achieved water is the only known substance (as of this date) that has a rest vibrational energy. No other known substance exhibits this peculiar behavior as it was assumed that at the absolute zero temperature every molecule would be totally frozen, having an energy of 0 J/mol. | ||
Additionally, water is usually referred to as "the universal solvent" because of its ability to dissolve more substances than any other existing liquid. Absolutely pure water theoretically has a neutral [[acidity]], which on a [[pH|pH scale]] has a value of practically 7.0 at 25˚C, but even traces of impurity can affect the pH. | Additionally, water is usually referred to as "the universal solvent" because of its ability to dissolve more substances than any other existing liquid. A [[Solution (chemistry)|solution]] in which water is the [[solvent]] is called an '''aqueous solution''', derived from the [[Latin]] ''aqua'' for water. Water is considered a [[Polar (chemistry)|polar]] solvent. Polar compounds are more likely to be miscible with (able to mix with) or dissolve in water, while non-polar compounds are immiscible with (do not mix with) or insoluble (or almost immiscible/insoluble) in water. Compounds of intermediate polarity can be partially miscible with or soluble in water. In [[chemical reaction]]s in dilute aqueous solutions, the [[concentration]] of liquid water is approximately constant at 55.5 [[Molarity|M]], so for simplicity the [[molarity]] of water is omitted from [[equilibrium constant]] expressions by convention. | ||
Liquid water molecules are constantly, readily, and rapidly dissociating to a slight extent to hydronium (H<sub>3</sub>O<sup>+</sup>) and [[hydroxide]] (OH<sup>-</sup>) ions as follows: | |||
:::2 H<sub>2</sub>O → H<sub>3</sub>O<sup>+</sup> + OH<sup>-</sup> | |||
and recombining in the reverse reaction. This reaction can be called dissociation or self-ionization of water. The dissociation (or ionization) constant for this reaction is symbolized as '''K<sub>w</sub>'''. At about 25°C, K<sub>w</sub> = 1 x 10<sup><small>-14</small></sup>. The expression for K<sub>w</sub> is: | |||
:::K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>] = 1 x 10<sup><small>-14</small></sup> | |||
Absolutely pure water theoretically has a neutral [[acidity]], which on a [[pH|pH scale]] has a value of practically 7.0 at 25˚C, but even traces of impurity can affect the pH. | |||
Frozen water has over 20 crystal structures it can assume dependent upon the circumstances. These water structures are denoted by Roman numbers, I -- XX. The structures of the different ice crystals are reflecting the response of water and its intramolecular interactions to the environment while freezing. This behavior also is specific for water, again making it in this respect an astonishing chemical. | Frozen water has over 20 crystal structures it can assume dependent upon the circumstances. These water structures are denoted by Roman numbers, I -- XX. The structures of the different ice crystals are reflecting the response of water and its intramolecular interactions to the environment while freezing. This behavior also is specific for water, again making it in this respect an astonishing chemical. | ||
Another special behavior of water is its internal charge distribution. Water is a polar liquid because of the electronegativity of [[Oxygen|O<sub>2</sub>]] compared to [[Hydrogen|H<sub>2</sub>]] making the | Another special behavior of water is its internal charge distribution. Water is a polar liquid because of the electronegativity of [[Oxygen|O<sub>2</sub>]] compared to [[Hydrogen|H<sub>2</sub>]] making the oxygen atom slightly negatively charged compared to the 2 hydrogen atoms in the water molecule. Since the electron clouds around oxygen are pyramidically shaped (compare '''visual''' with the diamond structure or the structure of methane). Oxygen can form a pyramidical temporary bond with adjacent water molecules. These quickly forming and dissolving bonds are called [[hydrogen bond|hydrogen-bridges]] because they are energetically substantial yet are formed and broken at a very high rate. These hydrogen-bridges are important in the behavior of water (in all phases). It is the main reason for the high boiling point (373.15 K) and the low freezing point (273.15 K) as well as the exceptional behavior that the [[Density (chemistry)|density]] of pure water is highest at 277.15 K (4˚C) where other liquid chemicals have the highest density at their freezing point. Sea water differs notably from pure water in that its density is greatest at its freezing point (about -1.9˚C). | ||
The exceptional capability of water to dissolve very many chemicals is for a large part due to the structure water can form, breaking and forming hydrogen-bridges all the time and is the major contributor to the biological importance of water. | The exceptional capability of water to dissolve very many chemicals is for a large part due to the structure water can form, breaking and forming hydrogen-bridges all the time and is the major contributor to the biological importance of water. | ||
===Unique properties of water=== | ===Unique properties of water=== | ||
Martin Chaplin has listed many of the unique properties exhibited by water.<ref> [ | Martin Chaplin has listed many of the unique properties exhibited by water.<ref> [https://doi.org/10.1002/9781119300762.wsts0002 Structure and Properties of Water in its Various States] Professor Martin Chaplin, [https://www.lsbu.ac.uk/ London South Bank University] (Last updated April 2021)</ref> | ||
Oceans, lakes, ponds, rivers, aquifers, and other natural liquid bodies of water on Earth can exist because water is liquid over a wide range of Earth's temperature, including its mean temperature of ~15<sup>o</sup>C. Icebergs exist because the density of solid water is lower than that of liquid water — ice floats.<ref name-orna1980>Orna MV. (1980) Water, the Peculiar Molecule. ''J. Chem. Ed.'' 57(12):891-892. | Oceans, lakes, ponds, rivers, aquifers, and other natural liquid bodies of water on Earth can exist because water is liquid over a wide range of Earth's temperature, including its mean temperature of ~15<sup>o</sup>C. Icebergs exist because the density of solid water is lower than that of liquid water — ice floats.<ref> name-orna1980>Orna MV. (1980) Water, the Peculiar Molecule. ''J. Chem. Ed.'' 57(12):891-892. ( Article written for secondary school chemistry.)</ref> | ||
==The biology perspective== | ==The biology perspective== | ||
{|align=" | {|align="center" style="width:50%; font-size: 98%;" | ||
| | | | ||
<font color="darkblue"><b>Water is life. It's the briny broth of our origins, the pounding circulatory system of the world, a precarious molecular edge on which we survive. It makes up two-thirds of our bodies, just like the map of the world; our vital fluids are saline, like the ocean. The apple doesn't fall far from the tree.</b><br> | <font color="darkblue"><b>Water is life. It's the briny broth of our origins, the pounding circulatory system of the world, a precarious molecular edge on which we survive. It makes up two-thirds of our bodies, just like the map of the world; our vital fluids are saline, like the ocean. The apple doesn't fall far from the tree.</b><br> | ||
'''— Barbara Kingsolver.'''</font><ref> Barbara Kingsolver. (2010) Water Is Life. National Geographic Magazine. April, 2010.</ref> | '''— Barbara Kingsolver.'''</font><ref> Barbara Kingsolver. (2010) Water Is Life. National Geographic Magazine. April, 2010.</ref> | ||
|} | |} | ||
On Earth, to the extent of present knowledge, nothing actively living, no [[Systems biology|system]] carrying out the universal commonalities of [[Life|living things]], can do so except in an aqueous medium, cellular and extracellular water. | |||
==Water by definition== | ==Water by definition== | ||
{{Image|Water drop.jpg|right|300px|Water, in its liquid state.}} | {{Image|Water drop.jpg|right|300px|Water, in its liquid state.}} | ||
{{Image|Drinking water.jpg|right|150px|Drinking water. | {{Image|Drinking water.jpg|right|150px|Drinking water.}} | ||
The word "water" itself is practically synonymous with the word "liquid", as we refer to different liquids as water-like | The word "water" itself is practically synonymous with the word "liquid", as we refer to different liquids as "water-like", "watered down" or "watery". We know that water moves and flows. When we come across another liquid which ''visibly resembles'' water with an unknown chemical makeup, we might infer that it is water but would not know until some analytical tests were performed. | ||
==Uses== | ==Uses== | ||
The availability of water on the Earth affords humanity an incredible number of uses, aside from consumption as an integral part of survival. Water can be used to cool machinery and facilities such as [[nuclear power plants]] and industrial milling tools. | The availability of water on the Earth affords humanity an incredible number of uses, aside from consumption as an integral part of survival. Water can be used to cool machinery and facilities such as [[nuclear power plants]] and industrial milling tools. Sometimes, water containing an isotope of hydrogen ([[Deuterium]]) and is referred to as "heavy water", is used for that same purpose of cooling. Water can also be heated to generate steam that can then be converted into power--the focus of the [[steam engine]] which was born out of the [[industrial revolution]]. Large dams use water flow and gravity to turn turbines and rotors to generate [[hydroelectric power]]. Water can also be pressurized, creating a narrow stream that can cut through concrete. | ||
===Bottled water=== | |||
In recent decades a multi-billion dollar market for "bottled water" has developed. This water is often advertised to have been additionally filtered, or to have come from a spring in order to enhance its "purity". However, there are cases where this was found to be untrue. The benefits and environmental impact of bottled water has been disputed for quite some time. Some governmental jurisdictions are concerned about the pollution created by the improper disposal of the plastic bottles and are considering a ban on the plastic throw-away bottles. However, some public health advocates agree that it is a healthy alternative to other drinks. | |||
==Quality== | ==Quality== | ||
| Line 125: | Line 139: | ||
In many parts of the world, water is obtained from a dug well, which draws from a deep pocket under the surface called an [[aquifer]]. Areas that use water from shallow aquifers for irrigation may exhibit a distinct rust discoloration due to iron oxidation. However, digging deeper may produce consumable water. The availability of water below the earth's surface and the depth at which it can be reached depends on the water table, which is directly impacted by the geological structure underneath. | In many parts of the world, water is obtained from a dug well, which draws from a deep pocket under the surface called an [[aquifer]]. Areas that use water from shallow aquifers for irrigation may exhibit a distinct rust discoloration due to iron oxidation. However, digging deeper may produce consumable water. The availability of water below the earth's surface and the depth at which it can be reached depends on the water table, which is directly impacted by the geological structure underneath. | ||
{{Image|Iron_oxide_deposit_from_well_water_irrigation.jpg| | {{Image|Iron_oxide_deposit_from_well_water_irrigation.jpg|right|250px|Iron oxide (rust) produced from a well-water irrigation system.}} | ||
In public groundwater and private water supplies in North America, it is desirable that fecal coliforms be virtually absent, while a level of under 10 per 100 ml is desirable in public surface water supplies. Permissible levels for total coliforms in recreational water are under 1,000 per 100 ml, and for fecal coliforms, under 100 per 100 ml<ref name=Prescott1996 />. Even very small numbers of pathogens are of concern, since high volumes of water may be consumed by humans. Contamination can result when [[sewage]] treatment systems break down, or when post-contamination occurs in pipelines. Against this possibility, residual [[chlorine]] is generally left in water after treatment. Such treatment, performed at a [[water treatment plant]], may include the processes of [[sedimentation]] ([[flocculation]]), [[filtration]], and [[disinfection]]<ref name=Prescott1996 /> with [[arsenic]], [[fluoride]], and other additives. (In the case of fluoride, the treatment may help prevent tooth decay.) Pathogens such as ''[[Giardiasis]]'' and ''[[Cryptosporidium]]'', however, produce [[cyst|cysts]] that are resistant to chlorination, and can only be removed by sedimentation and very fine filtration — through slow [[sand]] filters, for example<ref name=Prescott1996 />. | In public groundwater and private water supplies in North America, it is desirable that fecal coliforms be virtually absent, while a level of under 10 per 100 ml is desirable in public surface water supplies. Permissible levels for total coliforms in recreational water are under 1,000 per 100 ml, and for fecal coliforms, under 100 per 100 ml<ref name=Prescott1996 />. Even very small numbers of pathogens are of concern, since high volumes of water may be consumed by humans. Contamination can result when [[sewage]] treatment systems break down, or when post-contamination occurs in pipelines. Against this possibility, residual [[chlorine]] is generally left in water after treatment. Such treatment, performed at a [[water treatment plant]], may include the processes of [[sedimentation]] ([[flocculation]]), [[filtration]], and [[disinfection]]<ref name=Prescott1996 /> with [[arsenic]], [[fluoride]], and other additives. (In the case of fluoride, the treatment may help prevent tooth decay.) Pathogens such as ''[[Giardiasis]]'' and ''[[Cryptosporidium]]'', however, produce [[cyst|cysts]] that are resistant to chlorination, and can only be removed by sedimentation and very fine filtration — through slow [[sand]] filters, for example<ref name=Prescott1996 />. | ||
== | ==References== | ||
{{reflist}} | |||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 17:01, 6 November 2024
Water is a chemical compound composed of hydrogen and oxygen and has the chemical formula (H2O or HOH). At typical room temperature and pressure, it is a liquid that is nearly colorless, tasteless, and odorless. Its molecular structure and other properties are discussed in one of the sections below.
Water is the liquid substance that makes up the bulk of our oceans, lakes, and rivers. It is a liquid that animals, plants, and all living organisms must take in to keep living. It is the liquid we use to wash our bodies and our clothes and to put out fires. We learn early in life that water can be transformed into a solid called ice by freezing it or into water vapor by boiling it or allowing it to evaporate. We also realize that such transformations occur naturally, accounting for glaciers, icy roads, humid weather, clouds, rain, and snow. Pure gaseous water above its boiling point is called steam.
Scientists specializing in particular disciplines (e.g., physics, chemistry, geology, biology) study, describe and/or apply the properties and behaviors of water particular to their discipline. This article approaches those particulars in a multi-disciplinary, cross-disciplinary and inter-disciplinary way.
The earth sciences perspective
Oceans, seas, lakes, and rivers can be referred to as natural bodies of water. Oceans and seas consist of salt water, and so are considered saline. Many rivers and lakes consist of fresh water, meaning the water contains only trace amounts, if any, of salt. Water of intermediate salinity between salt water and fresh water is called brackish. Brackish water is commonly found where saline bodies of water meet bodies of fresh water. Among the many subjects dealt with by the earth sciences, water presents itself as a major focus and challenge.[1] In its broadest focus it deals with the distribution and masses of water on Earth. Oceanography deals with water in the oceans; hydrology, with water on land and underground; meteorology, with the characteristics of water in the Earth's atmosphere; glaciology, with water as glaciers and polar ice; climatology, with the role of water in Earth's climate.
Earth scientists also study and teach about the history of water in Earth's history as a planet, and unavoidably, about the possibility of water elsewhere in the Universe.
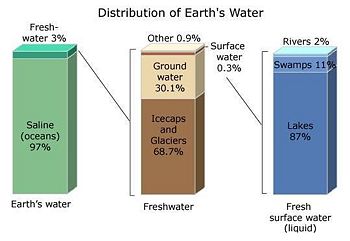
Water is one of the Earth's basic naturally occurring substances. It covers about 70% of the world's surface. If it covered the entire surface of a hypothetically flat Earth, the bottom of that flat 'waterworld' would lie 2.7 meters (8.9 feet) below the top.[2] In reality, only about 3% of Earth's water is fresh water, and of that about 30% is ground water, and about 70% is in ice caps, glaciers, and tundra (see accompanying illustration). Less than 1% of Earth's freshwater can be found in lakes, rivers, and clouds.
Humidity is the amount of water vapor present in the air. The amount of water vapor air can hold is highly dependent on its condition, typically including temperature. Water is constantly evaporating from bodies of water and entering the atmosphere to form clouds. Warm air can hold significantly more water vapor than cold air. When atmospheric conditions change such that the air can no longer hold the water vapor, precipitation occurs. Depending on temperature and conditions, precipitation can be rain in warmer weather, or snow, sleet, or hail in cold weather. Snow periodically falls on mountain tops forming glaciers. Glaciers continuously melt forming streams or creeks which flow together into rivers flowing downhill into other bodies of water, such as lakes, seas or oceans. Thus, a natural water cycle is ongoing.
The chemistry perspective
Chemistry deals with the properties of the water molecule itself, its interactions with other water molecules, and the way those properties and interactions account for its interactions with molecular species other than water — essentially all of its characteristics as 'water'.
The water molecule and its properties
|
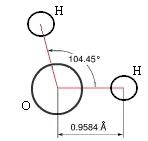
On a molecular level, water is a chemical compound, a 'molecule' comprised of two atoms of hydrogen bonded to one atom of oxygen (H2O) (see the two figures below]. The two types of atoms bond together into a single structure by sharing the unpaired electrons in the outermost electron shells surrounding their nuclei — i.e., by 'covalent' bonds. We can give the least technical explanation of the phenomenon in the following way. Atoms are electrically neutral, having equal numbers of protons (charged positively, situated in the central nucleus) and electrons (charged negatively, surrounding the nucleus in one or more 'shells'). A hydrogen atom has a nucleus with one proton and one (unpaired) electron in its only electron shell. An oxygen has a nucleus with eight protons, with its counterbalancing eight electrons distributed two in its first shell and six in its next and outermost shell — of the latter, two remaining unpaired. For reasons requiring considerations of quantum mechanics, a hydrogen atom behaves as if it 'needed' one more electron to pair up with its sole electron in its first (and only) electron shell, to fill that shell to its natural capacity. An oxygen atom has its first electron shell filled to capacity with two electrons, but behaves as if it 'needed' two additional electrons to pair up with the two unpaired electrons among the six in its next (and outermost) shell, which has a natural capacity of eight electrons. Two hydrogen atoms can thus share electrons with one oxygen atom, filling their respective outermost electron shells to capacity, achieving a more stable state in the covalent bonds.
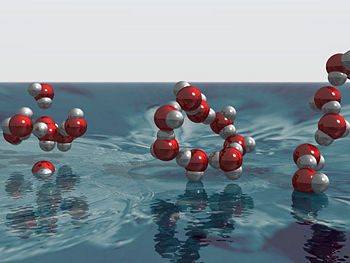
Three-dimensional model of individual water molecules associating with each other in three different structural configurations. Oxygen atoms shown in red, hydrogen atoms in grey.
The accompanying figures model the fact that the two hydrogen atoms do not bond symmetrically about the oxygen atom, but huddle somewhat closer together (~105o) than they would situated oppositely (180o) about the oxygen atom — the explanation of which requires knowledge of 'molecular orbital theory'. The plane of the molecule forms an isosceles triangle. Because the total charge of the nucleus of the larger oxygen atom exceeds that of the two smaller hydrogen atoms, the electrons spend more time nearer the oxygen atom, rendering the charge distribution on the molecule asymmetrical, with more negativity at the oxygen 'pole' of the molecule, and more positivity at the hydrogen 'pole' of the molecule. Chemists refer to the water molecule as 'polar', something roughly akin to the magnetic polar nature of a bar magnet. They also refer to the two covalent bonds in the molecule as 'polar covalent bonds'.
Though quantum mechanically simplistic, the above 'description' of the water molecule can serve to provide a fruitful level of understanding of the properties of water molecules in the aggregate, what we commonly refer to as the liquid, water, as ice, and as the gas, water vapor or steam — discussed in the next section.
Aggregates of water molecules and their properties
At one atmosphere of pressure, when liquid water is heated to 100 degrees Celsius (its boiling point), it begins to convert to steam, and when solid water — water ice — is heated to 0 degrees Celsius (its melting point) it begins to convert to liquid water. Water is unique among Earth's constituents in that it is the only naturally occurring substance that is found in those three states at temperatures normally occurring on Earth.
When cooled down to 0 K (absolute zero) or as close as can be practically achieved water is the only known substance (as of this date) that has a rest vibrational energy. No other known substance exhibits this peculiar behavior as it was assumed that at the absolute zero temperature every molecule would be totally frozen, having an energy of 0 J/mol.
Additionally, water is usually referred to as "the universal solvent" because of its ability to dissolve more substances than any other existing liquid. A solution in which water is the solvent is called an aqueous solution, derived from the Latin aqua for water. Water is considered a polar solvent. Polar compounds are more likely to be miscible with (able to mix with) or dissolve in water, while non-polar compounds are immiscible with (do not mix with) or insoluble (or almost immiscible/insoluble) in water. Compounds of intermediate polarity can be partially miscible with or soluble in water. In chemical reactions in dilute aqueous solutions, the concentration of liquid water is approximately constant at 55.5 M, so for simplicity the molarity of water is omitted from equilibrium constant expressions by convention.
Liquid water molecules are constantly, readily, and rapidly dissociating to a slight extent to hydronium (H3O+) and hydroxide (OH-) ions as follows:
- 2 H2O → H3O+ + OH-
and recombining in the reverse reaction. This reaction can be called dissociation or self-ionization of water. The dissociation (or ionization) constant for this reaction is symbolized as Kw. At about 25°C, Kw = 1 x 10-14. The expression for Kw is:
- Kw = [H3O+] [OH-] = 1 x 10-14
Absolutely pure water theoretically has a neutral acidity, which on a pH scale has a value of practically 7.0 at 25˚C, but even traces of impurity can affect the pH.
Frozen water has over 20 crystal structures it can assume dependent upon the circumstances. These water structures are denoted by Roman numbers, I -- XX. The structures of the different ice crystals are reflecting the response of water and its intramolecular interactions to the environment while freezing. This behavior also is specific for water, again making it in this respect an astonishing chemical.
Another special behavior of water is its internal charge distribution. Water is a polar liquid because of the electronegativity of O2 compared to H2 making the oxygen atom slightly negatively charged compared to the 2 hydrogen atoms in the water molecule. Since the electron clouds around oxygen are pyramidically shaped (compare visual with the diamond structure or the structure of methane). Oxygen can form a pyramidical temporary bond with adjacent water molecules. These quickly forming and dissolving bonds are called hydrogen-bridges because they are energetically substantial yet are formed and broken at a very high rate. These hydrogen-bridges are important in the behavior of water (in all phases). It is the main reason for the high boiling point (373.15 K) and the low freezing point (273.15 K) as well as the exceptional behavior that the density of pure water is highest at 277.15 K (4˚C) where other liquid chemicals have the highest density at their freezing point. Sea water differs notably from pure water in that its density is greatest at its freezing point (about -1.9˚C).
The exceptional capability of water to dissolve very many chemicals is for a large part due to the structure water can form, breaking and forming hydrogen-bridges all the time and is the major contributor to the biological importance of water.
Unique properties of water
Martin Chaplin has listed many of the unique properties exhibited by water.[5]
Oceans, lakes, ponds, rivers, aquifers, and other natural liquid bodies of water on Earth can exist because water is liquid over a wide range of Earth's temperature, including its mean temperature of ~15oC. Icebergs exist because the density of solid water is lower than that of liquid water — ice floats.[6]
The biology perspective
|
Water is life. It's the briny broth of our origins, the pounding circulatory system of the world, a precarious molecular edge on which we survive. It makes up two-thirds of our bodies, just like the map of the world; our vital fluids are saline, like the ocean. The apple doesn't fall far from the tree. |
On Earth, to the extent of present knowledge, nothing actively living, no system carrying out the universal commonalities of living things, can do so except in an aqueous medium, cellular and extracellular water.
Water by definition
The word "water" itself is practically synonymous with the word "liquid", as we refer to different liquids as "water-like", "watered down" or "watery". We know that water moves and flows. When we come across another liquid which visibly resembles water with an unknown chemical makeup, we might infer that it is water but would not know until some analytical tests were performed.
Uses
The availability of water on the Earth affords humanity an incredible number of uses, aside from consumption as an integral part of survival. Water can be used to cool machinery and facilities such as nuclear power plants and industrial milling tools. Sometimes, water containing an isotope of hydrogen (Deuterium) and is referred to as "heavy water", is used for that same purpose of cooling. Water can also be heated to generate steam that can then be converted into power--the focus of the steam engine which was born out of the industrial revolution. Large dams use water flow and gravity to turn turbines and rotors to generate hydroelectric power. Water can also be pressurized, creating a narrow stream that can cut through concrete.
Bottled water
In recent decades a multi-billion dollar market for "bottled water" has developed. This water is often advertised to have been additionally filtered, or to have come from a spring in order to enhance its "purity". However, there are cases where this was found to be untrue. The benefits and environmental impact of bottled water has been disputed for quite some time. Some governmental jurisdictions are concerned about the pollution created by the improper disposal of the plastic bottles and are considering a ban on the plastic throw-away bottles. However, some public health advocates agree that it is a healthy alternative to other drinks.
Quality
Water quality is generally determined by measuring the concentration of soluble chemicals, suspended clay particles, and microorganisms. With respect to human health, however, the most important criteria in measuring water quality is the degree of contamination by pathogenic microbes. Because natural water normally contains a large quantity of microorganisms that are nonpathogenic, the sanitary quality of water is determined by the kind, rather than number, of microorganisms.
Furthermore, water quality is measured only by the concentration of indicator bacteria, for measurement of all human pathogens would require too much time, and too many resources. Also, pathogens are difficult to detect due to their small and sporadically changing numbers in water, and their low survival rates (from exposure to water temperature, sunlight, chemicals, and other bacteria). An indicator microorganism is thus one that exists in large numbers in water, has stable properties and a longer survival time, and is absent from uncontaminated water[8].
These criteria are well satisfied when [[[coliform]]s are used as indicator microorganisms. Coliforms are Gram-negative, nonsporeforming, facultative (i.e. non-obligatory) anaerobic, rod-shaped bacteria that ferment lactose with the production of acid and gas within 48 hours at 35°C[8]. Two prominent coliforms are Escherichia coli and Enterobacter aerogenes, both enteric bacteria (that is, of the family Enterobacteriaceae). Along with viruses and protozoan pathogens such as Cryptosporidium, enteric bacteria are one of the major categories of waterborne disease of concern to humans[8]. Enteric bacteria, and in fact many pathogens, are transmitted by a fecal-oral route, and thus the presence of coliforms may indicate pollution by fecal material from humans (or other warmblooded animals).
To this end, coliforms are classified either as total coliforms, or the more specific fecal coliforms, which grow and ferment lactose with the production of acid and gas at 44.5 ± 0.2 °C for 24 ± 2 hours[8]. E. coli, indigenous to the human intestinal tract, is a fecal coliform, while Enterobacter aerogenes can also be found in soil and grain, and is thus considered a nonfecal coliform. Fecal counts are lower, and more clearly indicate fecal contamination by humans and other warmblooded animals than counts of total coliforms in water. E. coli, a major causative agent of the common "traveler's diarrhea," is the most commonly-used indicator microorganism in these counts[8].
Two accepted methods of determining coliform presence in water include the most-probable number (MPN) procedure, and the membrane filter technique. In the former, a series of tests is conducted until a positive result is reached, or a negative result is finally concluded in the last test. The tests involve looking for evidence of gas production under coliform incubation conditions, and observation of the colonies and cells of microorganisms in the water sample. The membrane filter technique uses filters that can select for either total or fecal coliforms, which form colonies on the pads. The more traditional MPN method has the principle advantage of not requiring any prior treatment of the water[8]. Advantages of the membrane filter method include a higher degree of reproducibility, more sensitivity (higher volumes of water can be tested), lower time requirements and fewer steps. The technique is disadvantaged in that the growth of the coliforms can be inhibited by background microorganisms such as cyanobacteria, and adsorbed metals and phenols, on the filter[8].
Treatment
In many parts of the world, water is obtained from a dug well, which draws from a deep pocket under the surface called an aquifer. Areas that use water from shallow aquifers for irrigation may exhibit a distinct rust discoloration due to iron oxidation. However, digging deeper may produce consumable water. The availability of water below the earth's surface and the depth at which it can be reached depends on the water table, which is directly impacted by the geological structure underneath.
In public groundwater and private water supplies in North America, it is desirable that fecal coliforms be virtually absent, while a level of under 10 per 100 ml is desirable in public surface water supplies. Permissible levels for total coliforms in recreational water are under 1,000 per 100 ml, and for fecal coliforms, under 100 per 100 ml[8]. Even very small numbers of pathogens are of concern, since high volumes of water may be consumed by humans. Contamination can result when sewage treatment systems break down, or when post-contamination occurs in pipelines. Against this possibility, residual chlorine is generally left in water after treatment. Such treatment, performed at a water treatment plant, may include the processes of sedimentation (flocculation), filtration, and disinfection[8] with arsenic, fluoride, and other additives. (In the case of fluoride, the treatment may help prevent tooth decay.) Pathogens such as Giardiasis and Cryptosporidium, however, produce cysts that are resistant to chlorination, and can only be removed by sedimentation and very fine filtration — through slow sand filters, for example[8].
References
- ↑ Gleick, Peter H. (2008) The World's Water 2008-2009: The Biennial Report on Freshwater Resources Includes chapters on: water and climate change; water in China; status of the Millennium Development Goals for water; peak water; efficient urban water use; business reporting on water....an updated chronology of global conflicts associated with water, as well as brief reviews of issues regarding desalination, the Salton Sea, and the Three Gorges Dam."
- ↑ Courtland R. (2008) Enough Water to Go Around? Nature News.
- ↑ For more information on why the freezing point of pure water is not measurable see:Revised Release on the Equation of State 2006 for H2O Ice Ih The International Association for the Properties of Water and Steam, The Netherlands, September 2009
- ↑ For more information on the colligative property of freezing point depression of water by adding of a solvent (such as a salt) see:Freezing Point Depression in Solutions Rod Nave, Department of Physics and Astronomy, Georgia State University
- ↑ Structure and Properties of Water in its Various States Professor Martin Chaplin, London South Bank University (Last updated April 2021)
- ↑ name-orna1980>Orna MV. (1980) Water, the Peculiar Molecule. J. Chem. Ed. 57(12):891-892. ( Article written for secondary school chemistry.)
- ↑ Barbara Kingsolver. (2010) Water Is Life. National Geographic Magazine. April, 2010.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 8.9 Prescott LM, Harley JP, Klein DA. 1996. Microbiology (3rd ed). Wm. C. Brown Publishers, Dubuque, Iowa. pp. 26, 107-108, 425, 428, 683, 770, 848-850, 852-853