Penicillin G: Difference between revisions
imported>David E. Volk (New page: {{subpages}} right|thumb|350px|{{#ifexist:Template:Penicillin G structure.jpg/credit|{{Penicillin G structure.jpg/credit}}<br/>|}}Penicillin G '''Pen...) |
mNo edit summary |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
'''Penicillin G''', a [[penicillin]] derivative, is a beta-[[lactam]]-based [[antibiotic]] drug used to treat severe infections from most | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Penicillin G structure.jpg|center|thumb|250px|{{#ifexist:Template:Penicillin G structure.jpg/credit|{{Penicillin G structure.jpg/credit}}<br/>|}}]] | |||
|width=250px | |||
|molname=penicillin G | |||
|synonyms='''benzopenicillin''', '''benzylpenicillin''' | |||
|molformula= C<sub>16</sub>H<sub>18</sub>N<sub>2</sub>O<sub>4</sub>S | |||
|molmass= 334.3901 | |||
|uses= antibiotic drug | |||
|properties= beta-lactam | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=61-33-6 | |||
}} | |||
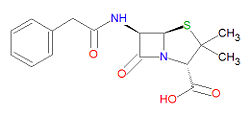
'''Penicillin G''', also called '''benzopenicillin''' or '''benzylpenicillin''', a [[penicillin]] derivative, is a beta-[[lactam]]-based [[antibiotic]] drug used to treat severe infections from most Gram-positive and some Gram-negative cocci. It is also an experimental drug for the treatment of convulsions due to it effects on synaptic transmission mediated by [[GABA|gamma-aminobutyric acid]]. | |||
== Chemistry == | == Chemistry == | ||
Penicillin G is | Penicillin G is degraded by (hydrolysis) by beta-[[lactamase]]s, including[[ penicillinase]]s, and [[cephalosporinase]]s. Its IUPAC chemical name is (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid and it has chemical formula C<sub>16</sub>H<sub>18</sub>N<sub>2</sub>O<sub>4</sub>S (MW = 334.3901 g/mol). | ||
== Mechanism of action == | == Mechanism of action == | ||
Penicillins work by binding to specific penicillin-binding proteins (PBPs) in the bacterial cell wall and inhibiting the final stage of bacterial cell wall synthesis, resulting in [[autolysis]] of the bacterial cells by [[autolysin]] enzymes. | Penicillins work by binding to specific penicillin-binding proteins (PBPs) in the bacterial cell wall and inhibiting the final stage of bacterial cell wall synthesis, resulting in [[autolysis]] of the bacterial cells by [[autolysin]] enzymes. | ||
== Drug interactions == | |||
Penicillins increase the effects of anticoagulant drugs including [[acenocoumarol]], [[anisindione]], [[dicumarol]] and [[warfarin]] and lessen the effects of some oral contraceptives including [[ethinyl estradiol]] and [[mestranol]]. [[Tetracycline]] and its derivatives [[doxycycline]], [[demeclocycline]], [[methacycline]], [[minocycline]], [[oxytetracycline]], and [[rolitetracycline]] may decrease the effectiveness of penicillin. The effects and toxicity of [[methotrexate]] increases when used with penicillins. | |||
== Brand names == | |||
{{col-begin}} | |||
{{col-break|width=33%}} | |||
# Abbocillin® | |||
# Ayercillin® | |||
# Benzopenicillin® | |||
# Benzylpenicillin® | |||
# Benzylpenicillin G® | |||
# Benzylpenicillinic Acid® | |||
# Bicillin® | |||
# Bicillin L-A® | |||
# Cillora® | |||
# Cilloral® | |||
# Cilopen® | |||
# Compocillin G® | |||
# Cosmopen® | |||
# Crysticillin 300 A.S.® | |||
{{col-break|width=33%}} | |||
# Dropcillin® | |||
# Free Benzylpenicillin® | |||
# Free Penicillin G® | |||
# Free Penicillin Ii® | |||
# Galofak® | |||
# Gelacillin® | |||
# Liquacillin® | |||
# Megacillin® | |||
# Pencillin G® | |||
# Penicillin® | |||
# Penicillin G Potassium® | |||
# Penicillin G Potassium in Plastic Container® | |||
# Penicillin G Sodium® | |||
# Penicillin-G Potassium® | |||
{{col-break|width=33%}} | |||
# Penicillinic Acid, Benzyl-® | |||
# Pentids® | |||
# Pentids '200'® | |||
# Permapen® | |||
# Pfizerpen® | |||
# Pfizerpen G® | |||
# Pharmacillin® | |||
# Phenylacetamidopenicillanic Acid® | |||
# Pradupen® | |||
# Specilline G® | |||
# Ursopen® | |||
# Wycillin® | |||
{{col-break|width=33%}} | |||
{{col-end}} | |||
== External links == | == External links == | ||
{{CZMed}} | |||
* Drug Bank [http://www.drugbank.ca/cgi-bin/getCard.cgi?CARD=DB01053.txt] | * Drug Bank [http://www.drugbank.ca/cgi-bin/getCard.cgi?CARD=DB01053.txt][[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 2 October 2024
|
| |||||||
| penicillin G | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Penicillin G, also called benzopenicillin or benzylpenicillin, a penicillin derivative, is a beta-lactam-based antibiotic drug used to treat severe infections from most Gram-positive and some Gram-negative cocci. It is also an experimental drug for the treatment of convulsions due to it effects on synaptic transmission mediated by gamma-aminobutyric acid.
Chemistry
Penicillin G is degraded by (hydrolysis) by beta-lactamases, includingpenicillinases, and cephalosporinases. Its IUPAC chemical name is (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid and it has chemical formula C16H18N2O4S (MW = 334.3901 g/mol).
Mechanism of action
Penicillins work by binding to specific penicillin-binding proteins (PBPs) in the bacterial cell wall and inhibiting the final stage of bacterial cell wall synthesis, resulting in autolysis of the bacterial cells by autolysin enzymes.
Drug interactions
Penicillins increase the effects of anticoagulant drugs including acenocoumarol, anisindione, dicumarol and warfarin and lessen the effects of some oral contraceptives including ethinyl estradiol and mestranol. Tetracycline and its derivatives doxycycline, demeclocycline, methacycline, minocycline, oxytetracycline, and rolitetracycline may decrease the effectiveness of penicillin. The effects and toxicity of methotrexate increases when used with penicillins.
Brand names
|
|
|
External links
The most up-to-date information about Penicillin G and other drugs can be found at the following sites.
- Penicillin G - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Penicillin G - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Penicillin G - Detailed information from DrugBank.
- Drug Bank [1]