Chloroform: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (add picture, fire hazard) |
mNo edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
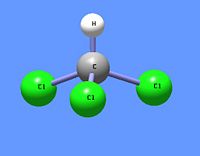
{{Image|Chloroform DEVolk.jpg|right|200px|Trichloromethane (chloroform) molecule CHCl<sub>3</sub>}} | |||
[[Chloroform]] (trichloromethane) is a chlorinated [[methane]] with three chlorine substituents. One of the first [[physician]]s to study and calculate dosages for the use of chloroform as surgical [[ | [[Chloroform]] ([[IUPAC]] name: '''trichloromethane''') is an [[Organic compound|organic]] [[chemical compound]] having the [[chemical formula]] CHCl<sub>3</sub>. It is a chlorinated [[methane]] with three [[chlorine]] substituents. At room [[temperature]] and [[pressure]], chloroform is a clear, colorless, somewhat [[volatile]] [[liquid]] with an odor characteristic of chlorinated hydrocarbons.* It has been commonly used as a fairly [[non-polar]] [[solvent]] in laboratories. | ||
<nowiki>*</nowiki> similar to the traditional old dry cleaners smell. That smell was [[perchloroethylene]]. | |||
==History== | |||
One of the first [[physician]]s to study and calculate dosages for the use of chloroform as surgical [[anesthesia]] was [[John Snow (physician)|John Snow]]. However, it was more [[toxic]] than [[diethyl ether]], another early anesthetic, and its use was discontinued.[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:01, 28 July 2024
Chloroform (IUPAC name: trichloromethane) is an organic chemical compound having the chemical formula CHCl3. It is a chlorinated methane with three chlorine substituents. At room temperature and pressure, chloroform is a clear, colorless, somewhat volatile liquid with an odor characteristic of chlorinated hydrocarbons.* It has been commonly used as a fairly non-polar solvent in laboratories.
* similar to the traditional old dry cleaners smell. That smell was perchloroethylene.
History
One of the first physicians to study and calculate dosages for the use of chloroform as surgical anesthesia was John Snow. However, it was more toxic than diethyl ether, another early anesthetic, and its use was discontinued.