Clausius-Clapeyron relation: Difference between revisions
imported>Paul Wormer No edit summary |
imported>Paul Wormer (I and II roman) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
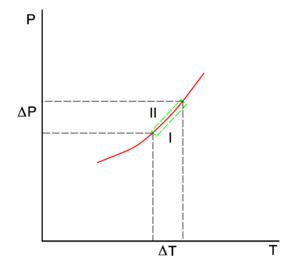
{{Image|Clapeyron.png|right|300px|The red line in the P-T diagram is the coexistence curve of two phases, | {{Image|Clapeyron.png|right|300px|The red line in the P-T diagram is the coexistence curve of two phases, I and II, of a single system. Phase II may be the vapor and I the liquid phase of the same compound. The lower green line gives the slope in phase I and the upper green line in phase II.}} | ||
The '''Clausius–Clapeyron relation''' is an equation for a system consisting of two phases of a single compound in thermodynamic equilibrium at constant absolute [[temperature]] ''T'' and constant [[pressure]] ''P''. A curve in a two-dimensional thermodynamical diagram that separates two phases in equilibrium is known as a [[coexistence curve]]. The Clausius–Clapeyron relation gives the [[slope]] of the coexistence curve in the ''P''-''T'' diagram: | The '''Clausius–Clapeyron relation''' is an equation for a system consisting of two phases of a single compound in thermodynamic equilibrium at constant absolute [[temperature]] ''T'' and constant [[pressure]] ''P''. A curve in a two-dimensional thermodynamical diagram that separates two phases in equilibrium is known as a [[coexistence curve]]. The Clausius–Clapeyron relation gives the [[slope]] of the coexistence curve in the ''P''-''T'' diagram: | ||
:<math> | :<math> | ||
\frac{\mathrm{d}P}{\mathrm{d}T} = \frac{Q}{T\,(V^{II} - V^{I})} , | \frac{\mathrm{d}P}{\mathrm{d}T} = \frac{Q}{T\,(V^{\mathrm{II}} - V^{\mathrm{I}})} , | ||
</math> | </math> | ||
where ''Q'' is the molar heat of transition. It is the heat necessary to bring one mole of the compound from phase | where ''Q'' is the molar heat of transition. It is the heat necessary to bring one mole of the compound from phase I into phase II; it is also known as ''latent heat''. For instance, when phase I is a liquid and phase II is a vapor, then ''Q'' ≡ ''H''<sub>v</sub> is the molar [[heat of vaporization]]. Further, ''V''<sup> I</sup> is the molar volume of phase I at the pressure ''P'' and temperature ''T'' of the point where the slope is considered and ''V''<sup> II</sup> is the same for phase II. | ||
The equation is named after [[Émile Clapeyron]], who derived it around 1834, and [[Rudolf Clausius]]. | The equation is named after [[Émile Clapeyron]], who derived it around 1834, and [[Rudolf Clausius]]. | ||
| Line 15: | Line 15: | ||
==Derivation== | ==Derivation== | ||
The condition of thermodynamical equilibrium at constant pressure ''P'' and constant | The condition of thermodynamical equilibrium at constant pressure ''P'' and constant | ||
temperature ''T'' between two phases | temperature ''T'' between two phases I and II is the equality of the molar [[Gibbs free energy|Gibbs free energies]] ''G'', | ||
:<math> | :<math> | ||
G^{I}(P | G^{\mathrm{I}}(T,P) = G^{\mathrm{II}}(T,P). \, | ||
</math> | </math> | ||
The molar Gibbs free energy of phase α (α = | The molar Gibbs free energy of phase α (α = I, II) is equal to the [[chemical potential]] | ||
μ<sup>α</sup> of this phase. Hence the equilibrium condition can be | μ<sup>α</sup> of this phase. Hence the equilibrium condition can be | ||
written as, | written as, | ||
:<math> \mu^{I}(P | :<math> \mu^{\mathrm{I}}(T,P) = \mu^{\mathrm{II}}(T,P), \; | ||
</math> | </math> | ||
which holds along the red | which holds everywhere along the coexistence (red) curve in the figure. | ||
If we go reversibly along the lower and upper green line in the figure, the chemical potentials of the phases change by Δμ<sup> | If we go reversibly along the lower and upper green line in the figure [the tangents to the curve in the point (''T'',''P'')], the chemical potentials of the phases, being functions of ''T'' and ''P'', change by Δμ<sup>I</sup> and Δμ<sup>II</sup>, for phase I and II, respectively, while the system stays in equilibrium, | ||
phase | |||
:<math> | :<math> | ||
\mu^{I}+\Delta \mu^{I} = \mu^{II}+\Delta \mu^{II} | \mu^{\mathrm{I}}+\Delta \mu^{\mathrm{I}} = \mu^{\mathrm{II}}+\Delta \mu^{\mathrm{II}} | ||
\;\Longrightarrow\; | \;\Longrightarrow\; | ||
\Delta \mu^{I} = \Delta \mu^{II} | \Delta \mu^{\mathrm{I}} = \Delta \mu^{\mathrm{II}} . | ||
</math> | </math> | ||
From classical thermodynamics it is known that | From classical thermodynamics it is known that | ||
:<math> | :<math> | ||
\Delta \mu^{\alpha}(P | \Delta \mu^{\alpha}(T,P) = \Delta G^{\alpha}(T,P)= -S^{\alpha} \Delta T | ||
+V^{\alpha} \Delta P, \quad\hbox{where}\quad \alpha = I, II. | +V^{\alpha} \Delta P, \quad\hbox{where}\quad \alpha = \mathrm{I, II}. | ||
</math> | </math> | ||
Here ''S''<sup>α</sup> is the molar entropy ([[entropy]] per [[mole]]) of phase | Here ''S''<sup>α</sup> is the molar entropy ([[entropy]] per [[mole]]) of phase | ||
| Line 42: | Line 42: | ||
phase. It follows that | phase. It follows that | ||
:<math> | :<math> | ||
-S^{I} \Delta T +V^{I} \Delta P = -S^{II} \Delta T +V^{II} \Delta P | -S^{\mathrm{I}} \Delta T +V^{\mathrm{I}} \Delta P = -S^{\mathrm{II}} \Delta T +V^{\mathrm{II}} \Delta P | ||
\;\Longrightarrow\; | \;\Longrightarrow\; | ||
\frac{\Delta P}{\Delta T} = \frac{S^{II} - S^{I}}{V^{II} - V^{I}} | \frac{\Delta P}{\Delta T} = \frac{S^{\mathrm{II}} - S^{\mathrm{I}}}{V^{\mathrm{II}} - V^{\mathrm{I}}}. | ||
</math> | </math> | ||
From the second law of thermodynamics it is known that for a reversible phase transition it holds that | From the second law of thermodynamics it is known that for a reversible phase transition it holds that | ||
:<math> | :<math> | ||
S^{II} - S^{I} = \frac{Q}{T} | S^{\mathrm{II}} - S^{\mathrm{I}} = \frac{Q}{T}, | ||
</math> | </math> | ||
where ''Q'' is the amount of heat necessary to convert one mole of | where ''Q'' is the amount of heat necessary to convert one mole of | ||
compound from phase | compound from phase I into phase II. Elimination of the entropy and taking the limit of infinitesimally small changes in ''T'' and ''P'' gives the ''Clausius-Clapeyron | ||
equation'', | equation'', | ||
:<math> | :<math> | ||
\frac{dP}{dT} = \frac{Q}{T(V^{II} - V^{I})} | \frac{dP}{dT} = \frac{Q}{T(V^{\mathrm{II}} - V^{\mathrm{I}})} . | ||
</math> | </math> | ||
==Approximate solution== | ==Approximate solution== | ||
| Line 60: | Line 60: | ||
The Clausius-Clapeyron equation is exact. When we make the following assumptions we may perform the integration: | The Clausius-Clapeyron equation is exact. When we make the following assumptions we may perform the integration: | ||
* The [[molar volume]] of phase | * The [[molar volume]] of phase I is negligible compared to the molar volume of phase II: ''V''<sup>II</sup> >> ''V''<sup>I</sup>. In general, far from the [[critical point]], this inequality holds well for liquid-gas equilibria. | ||
* Phase | * Phase II satisfies the [[ideal gas law]], where ''R'' is the [[molar gas constant]], | ||
::<math> | ::<math> | ||
PV^{II} = R T \, | PV^{\mathrm{II}} = R T. \, | ||
</math> | </math> | ||
:If phase | :If phase II is a gas and the pressure is fairly low, this assumption is reasonable. | ||
* The transition heat ''Q'' is constant over the temperature integration interval. The integrations run from the lower temperature ''T''<sub>1</sub> to the upper temperature ''T''<sub>2</sub> and from ''P''<sub>1</sub> to ''P''<sub>2</sub>. | * The transition (latent) heat ''Q'' is constant over the temperature integration interval. The integrations run from the lower temperature ''T''<sub>1</sub> to the upper temperature ''T''<sub>2</sub> and from ''P''<sub>1</sub> to ''P''<sub>2</sub>. | ||
Under these condition the Clausius-Clapeyron equation becomes | Under these condition the Clausius-Clapeyron equation becomes | ||
| Line 73: | Line 73: | ||
\frac{dP}{dT} = \frac{Q}{\frac{RT^2}{P}} | \frac{dP}{dT} = \frac{Q}{\frac{RT^2}{P}} | ||
\;\Longrightarrow\; | \;\Longrightarrow\; | ||
\frac{dP}{P} = \frac{Q}{R}\; \frac{dT}{T^2} | \frac{dP}{P} = \frac{Q}{R}\; \frac{dT}{T^2} . | ||
</math> | </math> | ||
Integration gives | Integration gives | ||
| Line 80: | Line 80: | ||
\frac{dT}{T^2} | \frac{dT}{T^2} | ||
\;\Longrightarrow\; | \;\Longrightarrow\; | ||
\ln\frac{P_2}{P_1} = -\frac{Q}{R}\;\left( \frac{1}{T_2} - \frac{1}{T_1} \right) | \ln\left(\frac{P_2}{P_1}\right) = -\frac{Q}{R}\;\left( \frac{1}{T_2} - \frac{1}{T_1} \right), | ||
</math> | </math> | ||
where ln is the natural (base ''e'') [[logarithm]]. We reiterate that for gas-liquid transitions ''Q'' | where ln(''P''<sub>2</sub>/''P''<sub>1</sub>) is the natural (base ''e'') [[logarithm]] of ''P''<sub>2</sub>/''P''<sub>1</sub>. We reiterate that for gas-liquid transitions ''Q'' = ''H''<sub>''v''</sub>, the [[heat of vaporization]]. | ||
Revision as of 02:30, 12 September 2009
The Clausius–Clapeyron relation is an equation for a system consisting of two phases of a single compound in thermodynamic equilibrium at constant absolute temperature T and constant pressure P. A curve in a two-dimensional thermodynamical diagram that separates two phases in equilibrium is known as a coexistence curve. The Clausius–Clapeyron relation gives the slope of the coexistence curve in the P-T diagram:
where Q is the molar heat of transition. It is the heat necessary to bring one mole of the compound from phase I into phase II; it is also known as latent heat. For instance, when phase I is a liquid and phase II is a vapor, then Q ≡ Hv is the molar heat of vaporization. Further, V I is the molar volume of phase I at the pressure P and temperature T of the point where the slope is considered and V II is the same for phase II.
The equation is named after Émile Clapeyron, who derived it around 1834, and Rudolf Clausius.
Derivation
The condition of thermodynamical equilibrium at constant pressure P and constant temperature T between two phases I and II is the equality of the molar Gibbs free energies G,
The molar Gibbs free energy of phase α (α = I, II) is equal to the chemical potential μα of this phase. Hence the equilibrium condition can be written as,
which holds everywhere along the coexistence (red) curve in the figure.
If we go reversibly along the lower and upper green line in the figure [the tangents to the curve in the point (T,P)], the chemical potentials of the phases, being functions of T and P, change by ΔμI and ΔμII, for phase I and II, respectively, while the system stays in equilibrium,
From classical thermodynamics it is known that
Here Sα is the molar entropy (entropy per mole) of phase α and Vα is the molar volume (volume of one mole) of this phase. It follows that
From the second law of thermodynamics it is known that for a reversible phase transition it holds that
where Q is the amount of heat necessary to convert one mole of compound from phase I into phase II. Elimination of the entropy and taking the limit of infinitesimally small changes in T and P gives the Clausius-Clapeyron equation,
Approximate solution
The Clausius-Clapeyron equation is exact. When we make the following assumptions we may perform the integration:
- The molar volume of phase I is negligible compared to the molar volume of phase II: VII >> VI. In general, far from the critical point, this inequality holds well for liquid-gas equilibria.
- Phase II satisfies the ideal gas law, where R is the molar gas constant,
- If phase II is a gas and the pressure is fairly low, this assumption is reasonable.
- The transition (latent) heat Q is constant over the temperature integration interval. The integrations run from the lower temperature T1 to the upper temperature T2 and from P1 to P2.
Under these condition the Clausius-Clapeyron equation becomes
Integration gives
where ln(P2/P1) is the natural (base e) logarithm of P2/P1. We reiterate that for gas-liquid transitions Q = Hv, the heat of vaporization.