Bariatric surgery: Difference between revisions
imported>Gareth Leng |
imported>Gareth Leng |
||
| Line 59: | Line 59: | ||
==Surgical Effects Regarding Gut Hormones== | ==Surgical Effects Regarding Gut Hormones== | ||
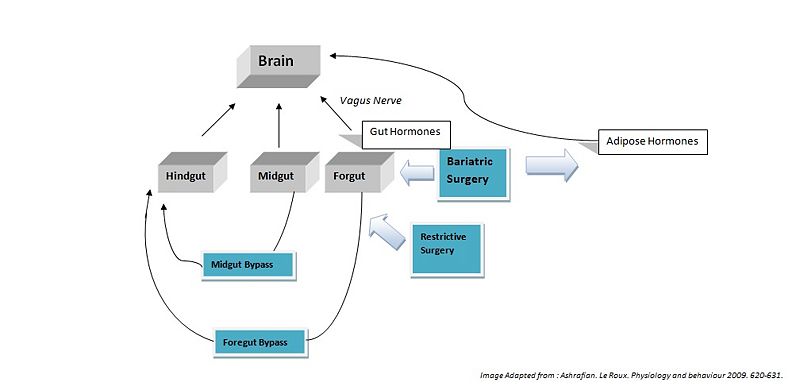
{{Image|Regulation of appetite.jpg|center|800px|''' | {{Image|Regulation of appetite.jpg|center|800px|'''Fig. 4'''. ''Bariatric surgery works by altering appetite though modulation of both gut hormones via the gut-brain axis and the adipose hormones via the adipose-brain axis. Restrictive surgery (gastric banding and sleeve gastrectomy) reduce the size of the [[foregut]]. Foregut bypass surgery (Gastric bypass and biliopancreatic diverson) bypasses anatomical segments of the [[stomach]] and [[duodenum]] (foregut) exposing the hindgut (posterior part of the large intestine). Midgut bypass surgery (jejuno-ileal bypass) bypasses anatomical segments of the [[jejunum]] and [[ileum]], exposing the hindgut''.}} | ||
===Hindgut Hormones[http://en.citizendium.org/wiki/Gut-brain_signalling]=== | ===Hindgut Hormones[http://en.citizendium.org/wiki/Gut-brain_signalling]=== | ||
'' | ''[[Peptide tyrosine tyrosine]]'' (PYY) is a peptide belonging to the [[neuropeptide Y]] (NPY) family <ref>(Broberger,2005)</ref>. PYY is normally released in proportion to calorie intake and has recently been described as an important feedback molecule in the Gut-Brain axis. As well as being a satiety signal, PPY has also been suggested to increase energy expenditure <ref>(Murphy)</ref>. Following bypass and restrictive surgery, basal and postprandial PYY levels increase <ref>Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation ''Physiology and Behaviour'' 97:620-31 [http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T0P-4VWB1BD-4-3&_cdi=4868&_user=10&_orig=search&_coverDate=07%2F14%2F2009&_sk=999029994&view=c&wchp=dGLzVtz-zSkzk&md5=3b8eb3b1242c0ef7fa254a9aa72f2d2b&ie=/sdarticle.pdf]</ref>. In addition, basal PYY levels increase following ileal resection, and patients have been reported to have lost weight (Broberger,2005). By contrast, diet-induced weight loss did not increase PYY levels <ref>(Olivan, 2009)</ref>. | ||
'' | ''[[Glucagon-like peptide-1]]'' (GLP-1), like PYY, as a circulating peptide hormone that acts on the hypothalamus as an anorexigenic signal. Most patients show a marked increase in basal and postprandial concentrations of GLP-1 after foregut bypass surgery. Midgut bypass also produces an increase in GLP-1 secretion, but studies on restrictive surgery have shown either no change or a decrease in basal levels of GLP-1 . Of specific interest GLP-1 has been shown to improve postprandial glycaemic control, being a potent incretin mimetic. A GLP-1 agonist, [[exenatide]], has not yet been approved for use as an anti-obesity drug, but it is already being used in the treatment of type 2 diabetes mellitus<ref>Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation ''Physiol Behav'' 97:620-31</ref>. | ||
===Midgut Hormones[http://en.citizendium.org/wiki/Gut-brain_signalling]=== | ===Midgut Hormones[http://en.citizendium.org/wiki/Gut-brain_signalling]=== | ||
'' | ''[[Neurotensin]]'' (NT), is a well know anorexigenic signal where its expression has been shown to be downregulated in ''ob/ob'' (leptin deficient) mice. Following bypass surgeries (5 of 6 reported) an increase in NT levels has been demonstrated which could contribute to the anorexigenic effects of the bypass operations<ref>Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation ''Physiol Behav'' 97:620-31</ref>. | ||
===Foregut Hormones[http://en.citizendium.org/wiki/Gut-brain_signalling]=== | ===Foregut Hormones[http://en.citizendium.org/wiki/Gut-brain_signalling]=== | ||
'' | ''[[Ghrelin]]'' is an orexigenic hormone released from the fundus of the [[stomach]]. It is released into the blood to promote eating, and levels begin to fall after meals when satiety is reached. Ghrelin is also believed to play a role in regulating body weight over a long period of time. As a result, ghrelin levels increase to compensate for the starvation induced by reduced calorie intake observed in diets. It would be assumed that bariatric surgery reduces hunger by lowering the amount of ghrelin released however the results from the literature remain controversial. Of 42 studies following foregut bypass surgeries, only 50% reported a decrease in ghrelin release. Many of these studies reported ghrelin levels immediately after surgery, whereas studies of long term ghrelin change following surgery show no significant trend. Results for restrictive surgeries show only an 18% decrease in ghrelin levels. These results indicated bariatric surgeries may not directly modulate ghrelin release <ref>Korner J ''et al.'' (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. ''J Clin Endocrinol Metab'' 90:359-65</ref> <ref>Holdstock C ''et al.'' (2003) Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. ''J Clin Endocrinol Metab'' 88:3177-83</ref>. | ||
'' | ''[[Cholecystokinin]]'' (CKK) was the first anorexigenic gut peptide to be implicated in the control of appetite <ref>(murphy)</ref>. One would speculate that CCK would rise following bariatric surgery however the results from the literature have been disappointing and suggest no role in the mediation of CCK following bariatric surgery <ref>Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation ''Physiol Behav'' 97:620-31</ref>. | ||
'' | ''[[Glucose-dependent Insulinotrophic Peptide]]'' (GIP) is a hormone produced in the foregut, and released when simple, energy-dense nutrients like glucose and [[free fatty acids]] are present and it promotes nutrient (energy) storage <ref>(Rosen, 2009)</ref>. Those suffering with obesity and diabetes often have a diet-induced, chronic elevation in GIP levels with peripheral tissue receptor down-regulation that is analogous to insulin insensitivity. Therefore, it would be optimal to decrease GIP levels and improve GIP sensitivity to lose weight. It has been showed that diet-induced weight loss increases GIP levels after a nutrient-rich meal and prevents further weight loss because the body counters the perceived starvation by increasing nutrient deposition in central and peripheral stores. By contrast, proximal foregut bypass surgery, roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch were found to decrease GIP levels and hence promote weight loss. | ||
==Surgical Treatment as Opposed to Diet== | ==Surgical Treatment as Opposed to Diet== | ||
Revision as of 06:10, 22 November 2009
This page was started in the framework of an Eduzendium course and needs to be assessed for quality. If this is done, this {{EZnotice}} can be removed.
Obesity is becoming a global pandemic whose manifestations are causing drastic consequences to our health and economy, and currently bariatric surgery is the most effective treatment for obesity as opposed to diet and drugs. In this article we shall discuss the specific bariatric operations and report how surgery effects hormonal modulation of both the gut-brain and adipose-brain axes as opposed to dieting. Our further understanding of how bariatric surgery works will yield novel pharmaceuticals in the treatment for metabolic disorders.
Introduction
Surgery as a treatment for obesity
For many severely obese patients, diets and lifestyle changes do not work in the long term. Bariatric surgery has proved to be the only effective method in the long term treatment of obesity. Bariatric surgery works by altering the anatomy of the gastrointestinal tract. This limits the volume of food the stomach can hold, interferes with the absorption of calories, and interferes with orexigenic and anorexigenic signalling between the gut, adipose tissues and the brain. Bariatric surgical procedures are continually evolving, and some of the procedures currently in use are describer here.
Bariatric Surgical Procedures
1. Restrictive Surgery
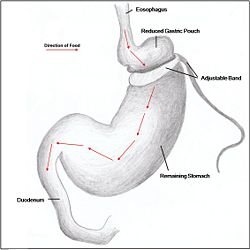
Restrictive surgery reduces the size of the stomach causing decreased hunger, and increased satiety following ingestion of food. Surgical procedures include gastric banding (Fig. 1), where an adjustable band is clipped just below the cardia of the stomach creating a small gastric pouch above (kerrigan, le roux). Other forms of restrictive surgery include gastroplasty, also called 'stomach stapling', and sleeve gastrectomy, where a large part of the stomach is surgically removed.
2. Malabsorptive Surgery
Jejuno-ileal bypass, duodenojejunal bypass and biliopancreatic bypass have, in the past, all been performed to reduce the amount of calories absorbed by reducing the size of the stomach. However, as a result of extreme malnourishment these procedures are now rarely performed. Instead, duodenal switches are the dominant form of malabsorptive surgery. Moreover, the procedure is usually accompanied with a restrictive form of surgery to have a greater effect on weight loss. Such forms of combination surgeries are most commonly performed.
3. Combination Surgery
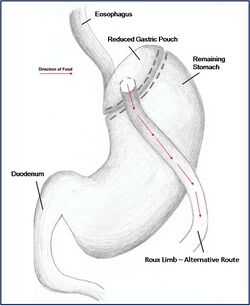
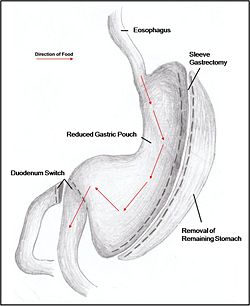
Combination surgery combines the benefits of both restrictive and malabsorptive surgery. The most common bariatric procedure in the USA is the Roux-en-Y Gastric Bypass (RYGP) (Fig. 2). Most restrictive procedures are performed alongside a duodenum switch. For instance, a sleeve gastrectomy reduces the stomach size drastically while the additional switch of the duodenum reduces the amount of calories absorbed (Fig. 3).
.
The Efficacy of Surgery
There is substantial weight loss following bariatric surgery. A meta-analysis of studies of 10,172 patients reported that, on average, patients lose 61.2% of their weight with bilopancreatic diversion and duodenal switch procedures ranking highest for weight loss efficacy (70.1% weight loss), followed closely by gastroplasty (68.2%) and gastric bypass (61.6%), with gastric banding ranked lowest (47.5%).
Bariatric surgery also improves a number of obesity related comorbidities which may prove to be of greater benefit than weight loss to the individual. An improvement or resolution of type 2 diabetes or impaired glucose tolerance was found following all types of bariatric surgeries. A resolution of diabetes allows patients to end their diabetes associated medication and improved glucose tolerance allows patients to maintain normal levels of blood glucose. For patients with diabetes before surgery, 76.8% resolved and 86% improved their diabetes with biliopancreatic diversion and duodenal switch procedures, as well ranking highest for weight loss efficacy, ranking highest for diabetes resolution (98.9% resolution), followed by gastric bypass (83.7%), gastroplasty (71.6%), and lastly gastric banding procedures (47.9%). [1]
Hyperlipidemia (high levels of lipids or lipoproteins in the blood), hypercholesterolemia (high blood cholesterol) and hypertriglyceridemia (high levels of triglycerides in blood) improve significantly in patients following all bariatric surgeries, with reported improvements of 70% or better [2]. Greatest improvements in these comorbidities are seen following biliopancreatic diversion, duodenal switch and gastric bypass procedures.
Hypertension (high blood pressure) improves following all bariatric surgeries. A meta-analysis study reported that 78.5% of patients show improvement in their hypertension and of 4805 patients studied, 61.7% in fact resolved their condition (Buchwald, 2004). The efficacy rankings of each surgery procedure are variable in terms of improvement or resolution.
Obstructive sleep apnea (a sleep disorder characterised by pauses in breathing caused by obstructed airways) and also obesity hypoventilation syndrome improves following all bariatric procedures with 85.7% of patients resolving their sleep apnea (Buchwald, 2004).
Surgical Effects Regarding Gut Hormones
Hindgut Hormones[2]
Peptide tyrosine tyrosine (PYY) is a peptide belonging to the neuropeptide Y (NPY) family [3]. PYY is normally released in proportion to calorie intake and has recently been described as an important feedback molecule in the Gut-Brain axis. As well as being a satiety signal, PPY has also been suggested to increase energy expenditure [4]. Following bypass and restrictive surgery, basal and postprandial PYY levels increase [5]. In addition, basal PYY levels increase following ileal resection, and patients have been reported to have lost weight (Broberger,2005). By contrast, diet-induced weight loss did not increase PYY levels [6].
Glucagon-like peptide-1 (GLP-1), like PYY, as a circulating peptide hormone that acts on the hypothalamus as an anorexigenic signal. Most patients show a marked increase in basal and postprandial concentrations of GLP-1 after foregut bypass surgery. Midgut bypass also produces an increase in GLP-1 secretion, but studies on restrictive surgery have shown either no change or a decrease in basal levels of GLP-1 . Of specific interest GLP-1 has been shown to improve postprandial glycaemic control, being a potent incretin mimetic. A GLP-1 agonist, exenatide, has not yet been approved for use as an anti-obesity drug, but it is already being used in the treatment of type 2 diabetes mellitus[7].
Midgut Hormones[3]
Neurotensin (NT), is a well know anorexigenic signal where its expression has been shown to be downregulated in ob/ob (leptin deficient) mice. Following bypass surgeries (5 of 6 reported) an increase in NT levels has been demonstrated which could contribute to the anorexigenic effects of the bypass operations[8].
Foregut Hormones[4]
Ghrelin is an orexigenic hormone released from the fundus of the stomach. It is released into the blood to promote eating, and levels begin to fall after meals when satiety is reached. Ghrelin is also believed to play a role in regulating body weight over a long period of time. As a result, ghrelin levels increase to compensate for the starvation induced by reduced calorie intake observed in diets. It would be assumed that bariatric surgery reduces hunger by lowering the amount of ghrelin released however the results from the literature remain controversial. Of 42 studies following foregut bypass surgeries, only 50% reported a decrease in ghrelin release. Many of these studies reported ghrelin levels immediately after surgery, whereas studies of long term ghrelin change following surgery show no significant trend. Results for restrictive surgeries show only an 18% decrease in ghrelin levels. These results indicated bariatric surgeries may not directly modulate ghrelin release [9] [10].
Cholecystokinin (CKK) was the first anorexigenic gut peptide to be implicated in the control of appetite [11]. One would speculate that CCK would rise following bariatric surgery however the results from the literature have been disappointing and suggest no role in the mediation of CCK following bariatric surgery [12].
Glucose-dependent Insulinotrophic Peptide (GIP) is a hormone produced in the foregut, and released when simple, energy-dense nutrients like glucose and free fatty acids are present and it promotes nutrient (energy) storage [13]. Those suffering with obesity and diabetes often have a diet-induced, chronic elevation in GIP levels with peripheral tissue receptor down-regulation that is analogous to insulin insensitivity. Therefore, it would be optimal to decrease GIP levels and improve GIP sensitivity to lose weight. It has been showed that diet-induced weight loss increases GIP levels after a nutrient-rich meal and prevents further weight loss because the body counters the perceived starvation by increasing nutrient deposition in central and peripheral stores. By contrast, proximal foregut bypass surgery, roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch were found to decrease GIP levels and hence promote weight loss.
Surgical Treatment as Opposed to Diet
It should be noted that this area of how bariatric surgery works is still quite controversial. Nevertheless, clinical observations demonstrate the effectiveness of surgery as opposed to diet. An attractive theory is as follows; when an individual consumes excessive amounts of calories satiety signals become abundant and their corresponding receptors become either desensitized or down regulated. Now when this obese individual attempts to lose weight by diet, satiety signals are ineffective. Thus the individual remains hungry. In addition in obese individuals following diets, body metabolism is so low that it makes dieting near to impossible. Bariatric surgery is proposed to work by increasing energy metabolism, reducing orexigenic signals, increasing anorexigenic signals and interfering with calorie absorption. For instance, after diet induced weight loss orexigenic hormones like ghrelin are released in abundance as the body is in 'starvation mode' - increasing the chances of the individual to over-eat to regain energy therefore regaining the weight. However, even though bariatric surgery similarly induces weight loss - the reduction in stomach size allows a proportionate decrease in ghrelin released. Hence, even though there is reduced calorie intake - the body does not try to compensate as less orexigenic signals are released. The precise functioning of these systems shall remain at the heart of controversy. Physiological reasoning is beyond the scope of this article however must also be considered [14].
Normally, obese people tend to have lower PYY levels compared to those who are lean, under fasting conditions. A year after bariatric surgery, such as jejunoileal bypass and vertical banding, the levels of fasting PYY was found to have increased. Moreover, concentrations within an hour of eating did not seem to change considerably - whether in an obese, lean, or postoperative. However, after Roux-en-Y gastric bypass, it has been reported that there is a postprandial PYY surge. There are no clear indications as to what might lead to this, however it is strongly believed that the reason for this exaggerated release is the removal of the pylorus (as well as a portion of the stomach) that allows food to enter the small intestine at a faster rate. This early rise in PYY levels will therefore also induce satiety early and reduce food intake. Again, even though such cases have in fact been reported - controversy still surrounds the exact mechanisms and affects surgery has on PYY levels due to the variability between individuals. There have not been enough postprandial PYY results from larger number of individuals that with strong statistical indications can correlate significant changes to satiety induction as a result of PYY level alterations post-operatively[15].
Type II Diabetes is usually developed in approximately 41% of obese cases as the body becomes resistant to insulin and inadequately responds to glucose - the correlative link between obesity and diabetes is now more commonly termed as Diabesity. Diet induced weight loss certainly ameliorates the insulin response but not to the extent where euglycemia is restored and insulin is normally released in response to glucose levels. Furthermore, the usual cases where weight is re-gained after diets will almost certainly lead to the re-establishment of insulin resistance and inadequate insulin response to glucose. After biliopancreatic diversion with Roux-en-Y gastric bypass, patients' insulin sensitivity had improved greatly, and its release according to glucose levels was also improved. It is important to take note that within a year after bariatric surgery, however massive the weight loss achieved, patients are usually still obese (with a BMI of ~ 30 kg/m2). However, the improvements of insulin resistance and its response to glucose are demonstrated to have taken place a few months after the operation. Therefore, the initial improvement of type 2 diabetes following bariatric surgery seems to take place even as the patient is still obese. This is a great asset to surgical weight loss as opposed to diet induced weight loss[16].
Future Possibilites and Conclusion
There is no doubt that further understanding of the physiology of how each specific operation effects hormonal modulation will lead to new pharmaceutical treatments in the treatment of obesity and appetite disorders.
Conclusion
References
- ↑ (Buchwald, 2004)
- ↑ (Buchwald et al, 2004)
- ↑ (Broberger,2005)
- ↑ (Murphy)
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiology and Behaviour 97:620-31 [1]
- ↑ (Olivan, 2009)
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31
- ↑ Korner J et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90:359-65
- ↑ Holdstock C et al. (2003) Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 88:3177-83
- ↑ (murphy)
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31
- ↑ (Rosen, 2009)
- ↑ Korner, J. et al. (2005). "Effects of Roux-en-Y Gastric Bypass Surgery on Fasting & Postprandial Concentrations of Plasma Ghrelin, Peptide YY, & Insulin". The Journal of Clinical Endocrinology and Metabolism 90(1):359-365
- ↑ Korner, J. et al. (2005). "Effects of Roux-en-Y Gastric Bypass Surgery on Fasting & Postprandial Concentrations of Plasma Ghrelin, Peptide YY, & Insulin". The Journal of Clinical Endocrinology and Metabolism 90(1):359-365
- ↑ Polyzogopoulou, E. V. et al. (2003). "Restoration of Euglycaemia and Normal Acute Insulin Response to Glucose in Obese Subjects with Type 2 Diabetes following Bariatric Surgery". Diabetes 52:1098-1103
Broberger, C. "Brain Regulation of food intake and appetite: molecules and networks" Journal of internal Medicine (2005); 258: 301-327.[5]
Buchwald, H. et al. "Bariatric surgery: A systematic review and meta-analysis" The Journal of American Medical Association (2004) 292(14): 1724-1737.[6]
DeMaria "Bariatric Surgery for Morbid Obesity". The new England Journal of Medicine (2007); 356; 2176-83 [7]
Murphy, K.G. "Gut Hormones in the control of appetite" Exp Physiol 89. pp 507-616. [8]