Randomized controlled trial/Citable Version: Difference between revisions
imported>Hayford Peirce |
imported>Hayford Peirce (saved copy from draft page) |
||
| Line 1: | Line 1: | ||
# | {{subpages}} | ||
{{TOC-right}} | |||
"A clinical trial is defined as a prospective scientific experiment that involves human subjects in whom treatment is initiated for the evaluation of a therapeutic intervention. In a '''randomized controlled clinical trial''', each patient is assigned to receive a specific treatment intervention by a chance mechanism."<ref name="pmid17339574">{{cite journal |author=Stanley K |title=Design of randomized controlled trials |journal=Circulation |volume=115 |issue=9 |pages=1164–9 |year=2007 |pmid=17339574 |doi=10.1161/CIRCULATIONAHA.105.594945}}</ref> The theory behind these trials is that the value of a treatment will be shown in an objective way, and, though usually unstated, there is an assumption that the results of the trial will be applicable to the care of patients who have the condition that was treated. | |||
Trials of potential treatments, for ethical reasons, tend to involve [[New Drug Application|multiple stages]], starting with small safety tests of the drug or other therapy. Once there is evidence of safety, and preliminary clinical trials, the effort moves to a larger scale: large multicentre clinical trials that are randomised, controlled, and double-blind using a [[#Control group|control group]] of patients (i.e., "arm" of the trial) and an experimental arm. | |||
Trials should be large, so that serious adverse events might be detected even when they occur rarely. Multi-centre trials minimise problems that can arise when a single geographical locus has a population that is not fully representative of the global population, and they can minimise the effect of geographical variations in environment and health care delivery. Randomisation (if the study population is large enough) should mean that the study groups are unbiased. A double-blind trial is one in which neither the patient nor the deliverer of the treatment is aware of the nature of the treatment offered to any particular individual, and this avoids bias caused by the expectations of either the doctor or the patient. | |||
Ethical trials exist in a framework. Before any work with human subjects begins, an appropriate [[institutional review board|independent review board]] evaluates the proposed experiment, the risks and benefits to subjects, and way in which [[informed consent]] will be given to participation. During the trial, an external safety board monitors progress; it has access to the actual, not double-blinded, data. If the experimental arm of the treatment, in the judgment of the board, is significantly more dangerous than existing therapy, the board can stop the trial. If the experimental treatment is strikingly better than the control arm, | |||
==Major developments in randomized controlled trials== | |||
In 1996, the [http://www.consort-statement.org/ Consort Statement] was published which improved how trials are reported.<ref name="pmid8773637">{{cite journal |author=Begg C, Cho M, Eastwood S, ''et al'' |title=Improving the quality of reporting of randomized controlled trials. The CONSORT statement |journal=JAMA |volume=276 |issue=8 |pages=637–9 |year=1996 |month=August |pmid=8773637 |doi= |url= |issn=}}</ref> The statement was revised in 2001.<ref name="pmid11308435">{{cite journal |author=Moher D, Schulz KF, Altman D |title=The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials |journal=JAMA |volume=285 |issue=15 |pages=1987–91 |year=2001 |month=April |pmid=11308435 |doi= |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=11308435 |issn=}}</ref> As of 2005, among high impact journals, "CONSORT was mentioned in the instructions of 36 (22%) journals (see bmj.com), more often in general and internal medicine journals (8/15; 53%) than in specialty journals (28/152; 18%)".<ref name="pmid15879389">{{cite journal |author=Altman DG |title=Endorsement of the CONSORT statement by high impact medical journals: survey of instructions for authors |journal=BMJ |volume=330 |issue=7499 |pages=1056–7 |year=2005 |month=May |pmid=15879389 |pmc=557224 |doi=10.1136/bmj.330.7499.1056 |url=http://bmj.com/cgi/pmidlookup?view=long&pmid=15879389 |issn=}}</ref> | |||
In 2004, the International Committee of Medical Journal Editors (ICMJE) announced that all trials starting enrollment after July 1, 2005 must be registered prior to consideration for publication in one of the 12 member journals of the Committee.<ref name="pmid15356289">{{cite journal |author=De Angelis C, Drazen JM, Frizelle FA, ''et al'' |title=Clinical trial registration: a statement from the International Committee of Medical Journal Editors |journal=The New England journal of medicine |volume=351 |issue=12 |pages=1250–1 |year=2004 |month=September |pmid=15356289 |doi=10.1056/NEJMe048225 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=15356289&promo=ONFLNS19 |issn=}}</ref> | |||
Again, clinical trials exist in a framework. When dealing with treatments, adverse effects may be discovered only after the end of the last phase, or in the larger populations to which a drug, approved for general release based on trial data, is used. [[Postmarketing surveillance|New Drug Application]] is intended to detect hazards that the trials are not powerful enough to find. | |||
==Control group== | |||
"Control", according to the current [[Declaration of Helsinki]] ethical guides, may or may not involve a [[placebo]]. If there is no accepted treatment, or the disease is mild and self-limiting, placebo controls may be ethical. If there is accepted treatment, the best accepted treatment becomes the control arm. Trials that are controlled, but not by a placebo, still generate arguments about information value versus ethics. | |||
Placebo controls are important, because the [[placebo effect]] can often be strong. The more value a subject believes an unknown drug has, but more placebo effect is has.<ref> Waber, Rebecca L., Baba Shiv, Ziv Carmon, and Dan Ariely. 2008. [http://jama.ama-assn.org/cgi/content/full/299/9/1016 Commercial Features of Placebo and Therapeutic Efficacy]. JAMA 299, no. 9:1016-1017.</ref> The use of historical rather than concurrent controls may lead to exagerated estimated of effect.<ref name="pmid7058834">{{cite journal |author=Sacks H, Chalmers TC, Smith H |title=Randomized versus historical controls for clinical trials |journal=Am. J. Med. |volume=72 |issue=2 |pages=233–40 |year=1982 |month=February |pmid=7058834 |doi= |url= |issn=}}</ref> | |||
Placebo effect can be seen in controlled trials of surgical interventions with the control group received a sham procedure.<ref name="pmid13657350">{{cite journal |author=Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA |title=An evaluation of internal-mammary-artery ligation by a double-blind technic |journal=N. Engl. J. Med. |volume=260 |issue=22 |pages=1115–8 |year=1959 |month=May |pmid=13657350 |doi= |url= |issn=}}</ref><ref name="pmid13816818">{{cite journal |author=Dimond EG, Kittle CF, Crockett JE |title=Comparison of internal mammary artery ligation and sham operation for angina pectoris |journal=Am. J. Cardiol. |volume=5 |issue= |pages=483–6 |year=1960 |month=April |pmid=13816818 |doi= |url= |issn=}}</ref><ref name="pmid12110735">{{cite journal |author=Moseley JB, O'Malley K, Petersen NJ, ''et al'' |title=A controlled trial of arthroscopic surgery for osteoarthritis of the knee |journal=N. Engl. J. Med. |volume=347 |issue=2 |pages=81–8 |year=2002 |month=July |pmid=12110735 |doi=10.1056/NEJMoa013259 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=12110735&promo=ONFLNS19 |issn=}}</ref> | |||
The Hawthorne effect is the improvements seen in control subjects simply from participating in research.<ref name="pmid17608932">{{cite journal |author=McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P |title=The Hawthorne Effect: a randomised, controlled trial |journal=BMC Med Res Methodol |volume=7 |issue= |pages=30 |year=2007 |pmid=17608932 |pmc=1936999 |doi=10.1186/1471-2288-7-30 |url=http://www.biomedcentral.com/1471-2288/7/30 |issn=}}</ref> | |||
==Variations in design== | |||
===Cluster-randomized trials=== | |||
In some settings, health care providers, or healthcare institutions should be randomized rather than randomizing the research subjects.<ref name="pmid-11927463">{{cite journal |author=Wears RL |title=Advanced statistics: statistical methods for analyzing cluster and cluster-randomized data |journal=Academic emergency medicine : official journal of the Society for Academic Emergency Medicine |volume=9 |issue=4 |pages=330–41 |year=2002 |pmid=11927463 |doi=}}</ref> This should occur when the intervention targets the provider or institutions and thus the results from each subject are not truly independent, but will cluster within the health care provider or healthcare institution. Guidelines exist for conducting cluster randomised trials.<ref name="pmid-15031246">{{cite journal |author=Campbell MK, Elbourne DR, Altman DG |title=CONSORT statement: extension to cluster randomised trials |journal=BMJ |volume=328 |issue=7441 |pages=702–8 |year=2004 |pmid=15031246 |doi=10.1136/bmj.328.7441.702|url=http://www.bmj.com/cgi/content/full/328/7441/702}}</ref> Cluster-randomized trials are not always correctly designed and executed.<ref> Eldridge, S., Ashby, D., Bennett, C., Wakelin, M., & Feder, G. (2008). Internal and external validity of cluster randomised trials: systematic review of recent trials. BMJ, http://www.bmj.com/cgi/content/full/bmj.39517.495764.25v1 {{doi| 10.1136/bmj.39517.495764.25}}.</ref> | |||
Designing an adequately sized cluster-randomized trial is based on several factors. One factor is the intraclass (intracluster) correlation coefficient (ICC).<ref name="pmid16279131">{{cite journal |author=Campbell MK, Fayers PM, Grimshaw JM |title=Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research |journal=Clin Trials |volume=2 |issue=2 |pages=99–107 |year=2005 |pmid=16279131 |doi=}}</ref><ref name="pmid10787581">{{cite journal |author=Campbell M, Grimshaw J, Steen N |title=Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action) |journal=J Health Serv Res Policy |volume=5 |issue=1 |pages=12–6 |year=2000 |pmid=10787581 |doi=}}</ref> The ICC between clusters in analogous to the variance between subject in a randomized controlled trial. Just as in Student's t-test for randomized controlled trial more variance between subjects means a larger study is needed, the less correlation between clusters means more clusters are needed. | |||
====Before-after studies==== | |||
Uncontrolled before-after studies and controlled before-after studies probably should not be considered variations of a randomized controlled trial, yet if carefully done offer advantages to observational studies.<ref name="pmid12810128">{{cite journal |author=Wyatt JC, Wyatt SM |title=When and how to evaluate health information systems? |journal=Int J Med Inform |volume=69 |issue=2-3 |pages=251–9 |year=2003 |pmid=12810128 |doi=10.1016/S1386-5056(02)00108-9 }}</ref> As in a true cluster-randomized trial, the intervention group can be randomly assigned; however, unlike a cluster-randomized trial, the before-after study does not have enough clusters or groups. An interrupted time series analysis can try to improve plausibility of causation; however, interrupted time series are commonly performed incorrectly.<ref name="pmid15095767">{{cite journal |author=Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE |title=Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies |journal=Int J Technol Assess Health Care |volume=19 |issue=4 |pages=613–23 |year=2003 |pmid=15095767 |doi=}}</ref> | |||
===Crossover trial=== | |||
In crossover trials, patients start in intervention and controls, but later all patients switch groups.<ref name="pmid9614025">{{cite journal |author=Sibbald B, Roberts C |title=Understanding controlled trials. Crossover trials |journal=BMJ |volume=316 |issue=7146 |pages=1719 |year=1998 |pmid=9614025 |doi= |issn=}}</ref> | |||
{| class="wikitable" align="right" | |||
|+ Factorial design | |||
!colspan="2" rowspan="2"| || colspan="2"| Intervention A | |||
|- | |||
| Given || Not given | |||
|- | |||
| rowspan="2"|'''Intervention B''' || Given || Group 1|| Group 2 | |||
|- | |||
| Not given|| Group 3|| Group 4 | |||
|} | |||
Variations on the standard AB, BA design have been proposed.<ref name="pmid6671121">{{cite journal |author=Laska E, Meisner M, Kushner HB |title=Optimal crossover designs in the presence of carryover effects |journal=Biometrics |volume=39 |issue=4 |pages=1087–91 |year=1983 |month=December |pmid=6671121 |doi= |url= |issn=}}</ref><ref name="pmid10091913">{{cite journal |author=Boon PC, Roes KC |title=Design and analysis issues for crossover designs in phase I clinical studies |journal=J Biopharm Stat |volume=9 |issue=1 |pages=109–28 |year=1999 |month=March |pmid=10091913 |doi= |url= |issn=}}</ref><ref name="pmid6733230">{{cite journal |author=Ebbutt AF |title=Three-period crossover designs for two treatments |journal=Biometrics |volume=40 |issue=1 |pages=219–24 |year=1984 |month=March |pmid=6733230 |doi= |url= |issn=}}</ref><ref name="pmid7889228">{{cite journal |author=Matthews JN |title=Multi-period crossover trials |journal=Stat Methods Med Res |volume=3 |issue=4 |pages=383–405 |year=1994 |month=December |pmid=7889228 |doi= |url= |issn=}}</ref> | |||
===Factorial design=== | |||
A factorial design allows two interventions to be be studied with ability to measure the treatment effect of each intervention in isolation and in combination.<ref name="pmid4023472">{{cite journal |author=Stampfer MJ, Buring JE, Willett W, Rosner B, Eberlein K, Hennekens CH |title=The 2 x 2 factorial design: its application to a randomized trial of aspirin and carotene in U.S. physicians |journal=Stat Med |volume=4 |issue=2 |pages=111–6 |year=1985 |pmid=4023472 |doi=10.1002/sim.4780040202 |url= |issn=}}</ref> | |||
===n of 1 trial=== | |||
In a "n of 1" trial, also called single-subject randomized trials, a single patient randomly proceeds through multiple blinded crossover comparisons. This address the concerns that traditional randomized controlled trials may not generalize to a specific patient.<ref name="pmid8616414">{{cite journal |author=Mahon J, Laupacis A, Donner A, Wood T |title=Randomised study of n of 1 trials versus standard practice |journal=BMJ |volume=312 |issue=7038 |pages=1069–74 |year=1996 |pmid=8616414 |doi= |issn=}}</ref> | |||
Underlining the difficulty in extrapolating from large trials to individual patients, Sackett proposed the use of N of 1 randomized controlled trials. In these, the patient is both the treatment group and the placebo group, but at different times. Blinding must be done with the collaboration of the pharmacist, and treatment effects must appear and disappear quickly following introduction and cessation of the therapy. This type of trial can be performed for many chronic, stable conditions.<ref name="pmid3409138">{{cite journal |author=Guyatt G ''et al'' |title=A clinician's guide for conducting randomized trials in individual patients |journal=CMAJ |volume=139 |pages=497–503 |year=1988 |pmid=3409138 |doi=}}</ref> The individualized nature of the single-subject randomized trial, and the fact that it often requires the active participation of the patient (questionnaires, diaries), appeals to the patient and promotes better insight and self-management<ref name="pmid17371593">{{cite journal |author=Brookes ST ''et al.'' |title="Me's me and you's you": Exploring patients' perspectives of single patient (n-of-1) trials in the UK |journal=Trials |volume=8 |pages=10 |year=2007 |pmid=17371593 |doi=10.1186/1745-6215-8-10}}</ref><ref name="pmid8299295">{{cite journal |author=Langer JC ''et al.'' |title=The single-subject randomized trial. A useful clinical tool for assessing therapeutic efficacy in pediatric practice |journal=Clinical Pediatrics |volume=32 |pages=654–7 |year=1993 |pmid=8299295 |doi=}}</ref> as well as patient safety,<ref name="pmid8616414">{{cite journal |author=Mahon J ''et al.'' |title=Randomised study of n of 1 trials versus standard practice |journal=BMJ |volume=312|pages=1069–74 |year=1996 |pmid=8616414 |doi=}}</ref> in a cost-effective manner. | |||
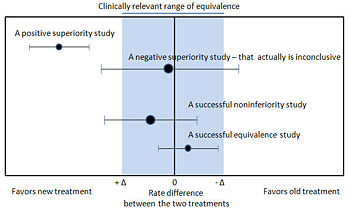
===Noninferiority and equivalence randomized trials=== | |||
<!--{|align="right" cellpadding="10" style="background-color:#FFFFCC; width:50%; border: 1px solid #aaa; margin:20px; font-size: 92%;" | |||
| | |||
''In the treatment of the sick person, the physician must be free to use a new diagnostic and therapeutic measure, if in his or her judgment it offers hope of saving life, re-establishing health or alleviating suffering. | |||
''The potential benefits, hazards and discomfort of a new method should be weighed against the advantages of the best current diagnostic and therapeutic methods. | |||
''In any medical study, every patient- including those of a control group, if any- should be assured of the best proven diagnostic and therapeutic method. | |||
''The physician can combine medical research with professional care, the objective being the acquisition of new medical knowledge,only to the extent that medical research is justified by its potential diagnostic or therapeutic value for the patient. | |||
From '''The Declaration of Helsinki'''<ref name="pmid14150898">{{cite journal |author=Rickham PP |title=Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki |journal=British Medical Journal |volume=2 |issue=5402 |pages=177 |year=1964 |month=July |pmid=14150898 |pmc=1816102 |doi= |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=Citizendium&pubmedid=14150898 |issn=}}</ref> | |||
|}--> | |||
{{Image|Noninferiority and equivalency randomized controlled trials.jpg|right|350px|Noninferiority and equivalency randomized controlled trials.}} | |||
As stated in The [[Declaration of Helsinki]] by the [[World Medical Association]] it is unethical to give any patient a placebo treatment if an existing treatment option is known to be beneficial.<ref>{{cite web |url=http://www.wma.net/e/policy/b3.htm |title=Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects |author=World Medical Association|accessdate=2007-11-17 |format= |work=}}</ref><ref name="pmid9062334">{{cite journal |author= |title=World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects |journal=JAMA |volume=277 |pages=925–6 |year=1997 |pmid=9062334 |doi=}}</ref> Many scientists and ethicists consider that the U.S. [[Food and Drug Administration]], by demanding placebo-controlled trials, encourages the systematic violation of the Declaration of Helsinki.<ref name="pmid12812185">{{cite journal |author=Michels KB, Rothman KJ |title=Update on unethical use of placebos in randomised trials |journal=Bioethics |volume=17 |pages=188–204 |year=2003 |pmid=12812185 |doi=}}</ref> In addition, the use of placebo controls remains a convenient way to avoid direct comparisons with a competing drug. | |||
The appropriate use of placebo is being revised.<ref name="pmid10975964">{{cite journal |author=Temple R, Ellenberg SS |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues |journal=Ann Intern Med |volume=133 |pages=455–63 |year=2000 |pmid=10975964 |doi=|url=http://www.annals.org/cgi/content/full/133/6/455}}</ref><ref name="pmid10975965">{{cite journal |author=Ellenberg SS, Temple R |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: practical issues and specific cases |journal=Ann Intern Med |volume=133 |pages=464–70 |year=2000 |pmid=10975965 |doi=|url=http://www.annals.org/cgi/content/full/133/6/464}}</ref> When guidelines suggest a placebo is an unethical control, then an "active-control noninferiority trial" may be used.<ref name="pmid16818930">{{cite journal |author=Kaul S, Diamond GA |title=Good enough: a primer on the analysis and interpretation of noninferiority trials |journal=Ann Intern Med |volume=145 |pages=62–9 |year=2006 |pmid=16818930 |doi=|url=http://www.annals.org/cgi/content/full/145/1/62}}</ref> To establish non-inferiority, the following three conditions should be - but frequently are not - established:<ref name="pmid16818930"/> | |||
# "The treatment under consideration exhibits therapeutic noninferiority to the active control." | |||
# "The treatment would exhibit therapeutic efficacy in a placebo-controlled trial if such a trial were to be performed." | |||
# "The treatment offers ancillary advantages in safety, tolerability, cost, or convenience." | |||
Noninferiority and equivalence randomized trial are difficult to execute well.<ref name="pmid16818930">{{cite journal |author=Kaul S, Diamond GA |title=Good enough: a primer on the analysis and interpretation of noninferiority trials |journal=Ann. Intern. Med. |volume=145 |issue=1 |pages=62–9 |year=2006 |pmid=16818930 |doi= |issn=}}</ref> Guidelines exists for noninferiority and equivalence randomized trials.<ref name="pmid16522836">{{cite journal |author=Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ |title=Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement |journal=JAMA |volume=295 |issue=10 |pages=1152–60 |year=2006 |pmid=16522836 |doi=10.1001/jama.295.10.1152 |issn=}}</ref> | |||
===Add-on design=== | |||
"Sometimes a new agent can be assessed by using an 'add-on' study design in which all patients | |||
are given standard therapy and are randomly assigned to also receive either new agent or placebo."<ref name="pmid10975964">{{cite journal |author=Temple R, Ellenberg SS |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues |journal=Ann Intern Med |volume=133 |pages=455–63 |year=2000 |pmid=10975964 |doi=|url=http://www.annals.org/cgi/content/full/133/6/455}}</ref> | |||
===Pragmatic trials=== | |||
[http://www.consort-statement.org/ Consort] has described pragmatic trials and standards for their reporting.<ref name="pmid19001484">{{cite journal |author=Zwarenstein M, Treweek S, Gagnier JJ, ''et al'' |title=Improving the reporting of pragmatic trials: an extension of the CONSORT statement |journal=BMJ |volume=337 |issue= |pages=a2390 |year=2008 |pmid=19001484 |doi= |url=http://bmj.com/cgi/pmidlookup?view=long&pmid=19001484 |issn=}}</ref> CONSORT describes a pragmatic trial as addressing "does the intervention work when used in normal practice" as opposed to an "explanatory trial" that addresses "can the intervention work." CONSORT states the there is not a dichotomy of trial designs, but that pragmatic reflects an attitude in the design. CONSORT offers an example of a pragmatic trial<ref name="pmid16980316">{{cite journal |author=Thomas KJ, MacPherson H, Thorpe L, ''et al'' |title=Randomised controlled trial of a short course of traditional acupuncture compared with usual care for persistent non-specific low back pain |journal=BMJ |volume=333 |issue=7569 |pages=623 |year=2006 |month=September |pmid=16980316 |pmc=1570824 |doi=10.1136/bmj.38878.907361.7C |url=http://bmj.com/cgi/pmidlookup?view=long&pmid=16980316 |issn=}}</ref> and an explanatory trial.<ref name="pmid1852179">{{cite journal |author= |title=Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators |journal=N. Engl. J. Med. |volume=325 |issue=7 |pages=445–53 |year=1991 |month=August |pmid=1852179 |doi= |url= |issn=}}</ref> | |||
==Ethical issues== | |||
The [[Declaration of Helsinki]] requires [[informed consent]] for participation in a trial. In the United States, there is an [[New drug application| approval procedure]] for clinical trials in human subjects, whether for research only or for potential approval of a commercial drug. Most industrialized countries have such procedures; some permit reciprocal approvals. | |||
===Ethics of randomizing subjects=== | |||
The appropriate use of placebo is being revised.<ref name="pmid10975964">{{cite journal |author=Temple R, Ellenberg SS |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues |journal=Ann Intern Med |volume=133 |pages=455–63 |year=2000 |pmid=10975964 |doi=|url=http://www.annals.org/cgi/content/full/133/6/455}}</ref><ref name="pmid10975965">{{cite journal |author=Ellenberg SS, Temple R |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: practical issues and specific cases |journal=Ann Intern Med |volume=133 |pages=464–70 |year=2000 |pmid=10975965 |doi=|url=http://www.annals.org/cgi/content/full/133/6/464}}</ref><ref name="pmid11565527">{{cite journal |author=Emanuel EJ, Miller FG |title=The ethics of placebo-controlled trials--a middle ground |journal=N. Engl. J. Med. |volume=345 |issue=12 |pages=915–9 |year=2001 |pmid=11565527 |doi=}}</ref> One tension is the balance between using placebo to increase scientific rigor versus the unnessessary deprival of active treatment to patients. This is the conundrum faced by Martin Arrowsmith in the book by the same name.<ref>{{Cite journal | doi = 10.1038/453038a | issn = 0028-0836 | volume = 453 | issue = 7191 | pages = 38 | last = Hausler | first = Thomas | title = In retrospect: When business became biology's plague | journal = Nature | accessdate = 2008-11-12 | date = 2008-05-01 | url = http://dx.doi.org/10.1038/453038a }}</ref><ref>{{Cite journal | doi = 10.1038/4531177b | issn = 0028-0836 | volume = 453 | issue = 7199 | pages = 1177 | last = Pastor | first = John | title = The ethical basis of the null hypothesis | journal = Nature | accessdate = 2008-11-12 | date = 2008-06-26 | url = http://dx.doi.org/10.1038/4531177b }}</ref> | |||
Comparing a new intervention to a placebo control may not be ethical when an accepted, effective treatment exists. In this case, the new intervention should be compared to the active control to establish whether the standard of care should change.<ref name="pmid8028622">{{cite journal |author=Rothman KJ, Michels KB |title=The continuing unethical use of placebo controls |journal=N. Engl. J. Med. |volume=331 |issue=6 |pages=394–8 |year=1994 |pmid=8028622 |doi=}}</ref> The observation that industry sponsored research may be more likely to conduct trials that have positive results suggest that industry is not picking the most appropriate comparison group.<ref name="pmid10968436">{{cite journal |author=Djulbegovic B, Lacevic M, Cantor A, ''et al'' |title=The uncertainty principle and industry-sponsored research |journal=Lancet |volume=356 |issue=9230 |pages=635–8 |year=2000 |pmid=10968436 |doi=}}</ref> However, it is possible that industry is better at predicting which new innovations are likely to be successful and discontinuing research for less promising interventions before the trial stage. | |||
There are times when placebo control is appropriate even when there is accepted, effective treatment.<ref name="pmid10975964">{{cite journal |author=Temple R, Ellenberg SS |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues |journal=Ann Intern Med |volume=133 |pages=455–63 |year=2000 |pmid=10975964 |doi=|url=http://www.annals.org/cgi/content/full/133/6/455}}</ref><ref name="pmid10975965">{{cite journal |author=Ellenberg SS, Temple R |title=Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: practical issues and specific cases |journal=Ann Intern Med |volume=133 |pages=464–70 |year=2000 |pmid=10975965 |doi=|url=http://www.annals.org/cgi/content/full/133/6/464}}</ref><ref name="pmid11565527">{{cite journal |author=Emanuel EJ, Miller FG |title=The ethics of placebo-controlled trials--a middle ground |journal=N. Engl. J. Med. |volume=345 |issue=12 |pages=915–9 |year=2001 |pmid=11565527 |doi=}}</ref> | |||
There are ethical concerns in comparing a surgical intervention to sham surgery; however, this has been done.<ref>Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA: An evaluation of internal-mammary-artery ligation by a double-blind technique. N Engl J Med 1959;260:1115-1118.</ref><ref name="pmid12110735">{{cite journal |author=Moseley JB, O'Malley K, Petersen NJ, ''et al'' |title=A controlled trial of arthroscopic surgery for osteoarthritis of the knee |journal=N. Engl. J. Med. |volume=347 |issue=2 |pages=81–8 |year=2002 |pmid=12110735 |doi=10.1056/NEJMoa013259}}</ref> Guidelines by the [[American Medical Association]] address the use of placebo surgery.<ref name="pmid11807373">{{cite journal |author=Tenery R, Rakatansky H, Riddick FA, ''et al'' |title=Surgical "placebo" controls |journal=Ann. Surg. |volume=235 |issue=2 |pages=303–7 |year=2002 |pmid=11807373 |doi=}}</ref> | |||
===Interim analysis - stopping trials early=== | |||
Trials are increasingly stopped early<ref name="pmid16264162">{{cite journal |author=Montori VM, Devereaux PJ, Adhikari NK, ''et al'' |title=Randomized trials stopped early for benefit: a systematic review |journal=JAMA |volume=294 |issue=17 |pages=2203–9 |year=2005 |month=November |pmid=16264162 |doi=10.1001/jama.294.17.2203 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=16264162 |issn=}}</ref>; however, this may induce a bias. Data safety and monitoring boards that are independent of the trial are commissioned to conduct interim analyses and make decisions about stopping trials early.<ref>Trotta, F., G. Apolone, S. Garattini, and G. Tafuri. 2008. Stopping a trial early in oncology: for patients or for industry? Ann Oncol mdn042. http://dx.doi.org/10.1093/annonc/mdn042</ref> <ref name="pmid15014189">{{cite journal |author=Slutsky AS, Lavery JV |title=Data Safety and Monitoring Boards |journal=N. Engl. J. Med. |volume=350 |issue=11 |pages=1143–7 |year=2004 |month=March |pmid=15014189 |doi=10.1056/NEJMsb033476 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=15014189&promo=ONFLNS19 |issn=}}</ref> | |||
Reasons to stop a trial early are efficacy, safety, and futility.<ref name="pmid18398083">{{cite journal |author=Borer JS, Gordon DJ, Geller NL |title=When should data and safety monitoring committees share interim results in cardiovascular trials? |journal=JAMA |volume=299 |issue=14 |pages=1710–2 |year=2008 |month=April |pmid=18398083 |doi=10.1001/jama.299.14.1710 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=18398083 |issn=}}</ref><ref name="pmid18226746">{{cite journal |author=Bassler D, Montori VM, Briel M, Glasziou P, Guyatt G |title=Early stopping of randomized clinical trials for overt efficacy is problematic |journal=J Clin Epidemiol |volume=61 |issue=3 |pages=241–6 |year=2008 |month=March |pmid=18226746 |doi=10.1016/j.jclinepi.2007.07.016 |url=http://linkinghub.elsevier.com/retrieve/pii/S0895-4356(07)00292-2 |issn=}}</ref> | |||
Regarding efficacy, various rules exist that adjust alpha to decide when to stop a trial early.<ref name="pmid16264167">{{cite journal |author=Pocock SJ |title=When (not) to stop a clinical trial for benefit |journal=JAMA |volume=294 |issue=17 |pages=2228–30 |year=2005 |month=November |pmid=16264167 |doi=10.1001/jama.294.17.2228 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=16264167 |issn=}}</ref><ref name="pmid15885299">{{cite journal |author=Schulz KF, Grimes DA |title=Multiplicity in randomised trials II: subgroup and interim analyses |journal=Lancet |volume=365 |issue=9471 |pages=1657–61 |year=2005 |pmid=15885299 |doi=10.1016/S0140-6736(05)66516-6 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(05)66516-6 |issn=}}</ref><ref name="pmid15345605">{{cite journal |author=Grant A |title=Stopping clinical trials early |journal=BMJ |volume=329 |issue=7465 |pages=525–6 |year=2004 |month=September |pmid=15345605 |doi=10.1136/bmj.329.7465.525 |url=http://bmj.com/cgi/pmidlookup?view=long&pmid=15345605 |issn=}}</ref><ref name="pmid497341">{{cite journal |author=O'Brien PC, Fleming TR |title=A multiple testing procedure for clinical trials |journal=Biometrics |volume=35 |issue=3 |pages=549–56 |year=1979 |month=September |pmid=497341 |doi= |url= |issn=}}</ref><ref name="pmid7786985">{{cite journal |author=Bauer P, Köhne K |title=Evaluation of experiments with adaptive interim analyses |journal=Biometrics |volume=50 |issue=4 |pages=1029–41 |year=1994 |month=December |pmid=7786985 |doi= |url= |issn=}} This method was used by PMID: 18184958</ref> A commonly recommended rules are the O'Brien-Fleming (the O'Brien-Fleming rule requires a varying p-value depending on the number of interim analyses) and the Haybittle-Peto (the Haybittle-Peto which requires p<0.001 to stop a trial early) rule.<ref name="pmid16264167"/><ref name="pmid15885299"/><ref name="isbn0-471-48986-7">{{cite book |author=DeMets, David L.; Susan S. Ellenberg; Fleming, Thomas J. |authorlink= |editor= |others= |title=Data Monitoring Committees in Clinical Trials: a Practical Perspective |edition= |language= |publisher=J. Wiley & Sons |location=New York |year=2002 |origyear= |pages= |quote= |isbn=0-471-48986-7 |oclc= |doi= |url=http://books.google.com/books?isbn=0471489867 |accessdate=}}</ref> | |||
Using a more conservative stopping rule reduces the chance of a statistical alpha (Type I) error; however, these rules do not alter that the effect size may be exaggerated.<ref name="pmid2605969">{{cite journal |author=Pocock SJ, Hughes MD |title=Practical problems in interim analyses, with particular regard to estimation |journal=Control Clin Trials |volume=10 |issue=4 Suppl |pages=209S–221S |year=1989 |month=December |pmid=2605969 |doi= |url= |issn=}}</ref><ref name="pmid15885299">{{cite journal |author=Schulz KF, Grimes DA |title=Multiplicity in randomised trials II: subgroup and interim analyses |journal=Lancet |volume=365 |issue=9471 |pages=1657–61 |year=2005 |pmid=15885299 |doi=10.1016/S0140-6736(05)66516-6 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(05)66516-6 |issn=}}</ref> According to Bassler, "the more stringent the P-value threshold results must cross to justify stopping the trial, the more likely it is that a trial stopped early will overestimate the treatment effect."<ref name="pmid18226746">{{cite journal |author=Bassler D, Montori VM, Briel M, Glasziou P, Guyatt G |title=Early stopping of randomized clinical trials for overt efficacy is problematic |journal=J Clin Epidemiol |volume=61 |issue=3 |pages=241–6 |year=2008 |month=March |pmid=18226746 |doi=10.1016/j.jclinepi.2007.07.016 |url=http://linkinghub.elsevier.com/retrieve/pii/S0895-4356(07)00292-2 |issn=}}</ref> A review of trials stopped early found that the earlier a trial was stopped the larger was its reported treatment effect.<ref name="pmid16264162">{{cite journal |author=Montori VM, Devereaux PJ, Adhikari NK, ''et al'' |title=Randomized trials stopped early for benefit: a systematic review |journal=JAMA |volume=294 |issue=17 |pages=2203–9 |year=2005 |month=November |pmid=16264162 |doi=10.1001/jama.294.17.2203 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=16264162 |issn=}}</ref> Accordingly, examples exists of trials whose interim analyses were significant, but the trial was continued and the final analysis was less significant or was insignificant.<ref name="pmid15894981">{{cite journal |author=Pocock S, Wang D, Wilhelmsen L, Hennekens CH |title=The data monitoring experience in the Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) program |journal=Am. Heart J. |volume=149 |issue=5 |pages=939–43 |year=2005 |month=May |pmid=15894981 |doi=10.1016/j.ahj.2004.10.038 |url=http://linkinghub.elsevier.com/retrieve/pii/S0002870305000189 |issn=}}</ref><ref name="pmid12559643">{{cite journal |author=Wheatley K, Clayton D |title=Be skeptical about unexpected large apparent treatment effects: the case of an MRC AML12 randomization |journal=Control Clin Trials |volume=24 |issue=1 |pages=66–70 |year=2003 |month=February |pmid=12559643 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0197245602002738 |issn=}}</ref><ref name="pmid12851279">{{cite journal |author=Abraham E, Reinhart K, Opal S, ''et al'' |title=Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial |journal=JAMA |volume=290 |issue=2 |pages=238–47 |year=2003 |month=July |pmid=12851279 |doi=10.1001/jama.290.2.238 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=12851279 |issn=}}</ref> Methods to correct for exaggeration exists.<ref name="pmid3231947">{{cite journal |author=Hughes MD, Pocock SJ |title=Stopping rules and estimation problems in clinical trials |journal=Stat Med |volume=7 |issue=12 |pages=1231–42 |year=1988 |month=December |pmid=3231947 |doi= |url= |issn=}}</ref><ref name="pmid2605969">{{cite journal |author=Pocock SJ, Hughes MD |title=Practical problems in interim analyses, with particular regard to estimation |journal=Control Clin Trials |volume=10 |issue=4 Suppl |pages=209S–221S |year=1989 |month=December |pmid=2605969 |doi= |url= |issn=}}</ref> | |||
As an alternative to the alpha rules, conditional power can help decide when to stop trials early.<ref name="isbn0-387-27781-1">{{cite book |author= |authorlink= |editor= |others= |title=Statistical Monitoring of Clinical Trials: Fundamentals for Investigators |edition= |language= |publisher=Springer |location=Berlin |year=2005 |origyear= |pages= |quote= |isbn=0-387-27781-1 |oclc= |doi= |url= |accessdate=}}</ref><ref name="pmid16345019">{{cite journal |author=Lachin JM |title=Operating characteristics of sample size re-estimation with futility stopping based on conditional power |journal=Stat Med |volume=25 |issue=19 |pages=3348–65 |year=2006 |month=October |pmid=16345019 |doi=10.1002/sim.2455 |url=http://dx.doi.org/10.1002/sim.2455 |issn=}}</ref> | |||
===Seeding trials=== | |||
Seeding trials are studies sponsored by industry whose "apparent purpose is to test a hypothesis. The true purpose is to get physicians in the habit of prescribing a new drug."<ref name="pmid18711155">{{cite journal |author=Hill KP, Ross JS, Egilman DS, Krumholz HM |title=The ADVANTAGE seeding trial: a review of internal documents |journal=Ann. Intern. Med. |volume=149 |issue=4 |pages=251–8 |year=2008 |month=August |pmid=18711155 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=long&pmid=18711155 |issn=}}</ref> | |||
==Measuring outcomes== | |||
Subjectively assessed outcomes are more susceptible to bias in trials with inadequate allocation concealment.<ref>{{Cite journal | |||
| doi = 10.1136/bmj.39465.451748.AD | volume = 336 | issue = 7644 | pages = 601-605 | last = Wood | first = Lesley | coauthors = Matthias Egger, Lise Lotte Gluud, Kenneth F Schulz, Peter Juni, Douglas G Altman, Christian Gluud, Richard M Martin, Anthony J G Wood, Jonathan A C Sterne | title = Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study | journal = BMJ | accessdate = 2008-03-14 | date = 2008-03-15 | url = http://www.bmj.com/cgi/content/abstract/336/7644/601}}</ref> | |||
===Missing data=== | |||
Missing data created decisions regarding what outcome to assign to that patient and what experimental group to assign the patient to. | |||
* Regarding assigning an outcome to the patient, using a 'last observation carried forward' (LOCF) analysis may introduce biases.<ref name="pmid2381523">{{cite journal |author=Hauser WA, Rich SS, Annegers JF, Anderson VE |title=Seizure recurrence after a 1st unprovoked seizure: an extended follow-up |journal=Neurology |volume=40 |issue=8 |pages=1163–70 |year=1990 |month=August |pmid=2381523 |doi= |url= |issn=}}</ref> | |||
* Regarding group assignment, a 'per protocol' analysis may introduce bias compared to an 'intention to treat' analysis. | |||
===Surrogate outcomes=== | |||
The costs and efforts required to measure primary endpoints such as morbidity and mortality make using surrogate outcomes an option. An example is in the treatment of osteoporosis, the primary outcomes are fractures and mortality whereas the surrogate outcome is changes in [[bone mineral density]].<ref name="pmid15615079">{{cite journal |author=Li Z, Chines AA, Meredith MP |title=Statistical validation of surrogate endpoints: is bone density a valid surrogate for fracture? |journal=J Musculoskelet Neuronal Interact |volume=4 |issue=1 |pages=64–74 |year=2004 |pmid=15615079 |doi=10.1081/BIP-120024209|url=http://www.ismni.org/jmni/pdf/15/09ZLI.pdf}}</ref><ref name="pmid14584722">{{cite journal |author=Li Z, Meredith MP |title=Exploring the relationship between surrogates and clinical outcomes: analysis of individual patient data vs. meta-regression on group-level summary statistics |journal=J Biopharm Stat |volume=13 |issue=4 |pages=777–92 |year=2003 |pmid=14584722 |doi=10.1081/BIP-120024209}}</ref> Other examples of surrogate outcomes are tumor shrinkage or changes in cholesterol level, blood pressure, HbA1c, CD4 cell count.<ref name="pmid8815760">{{cite journal |author=Fleming TR, DeMets DL |title=Surrogate end points in clinical trials: are we being misled? |journal=Ann. Intern. Med. |volume=125 |issue=7 |pages=605–13 |year=1996 |pmid=8815760 |doi=|url=http://www.annals.org/cgi/content/full/125/7/605}}</ref> Surrogate markers might be acceptable when "the surrogate must be a correlate of the true clinical outcome and fully capture the net effect of treatment on the clinical outcome".<ref name="pmid8815760"/> | |||
===Composite outcomes=== | |||
Composite outcomes are problematic if the components of the composite vary in their magnitude and contribution to the composite.<ref name="pmid17403713">{{cite journal |author=Ferreira-González I, Busse JW, Heels-Ansdell D, ''et al'' |title=Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials |journal=BMJ |volume=334 |issue=7597 |pages=786 |year=2007 |month=April |pmid=17403713 |pmc=1852019 |doi=10.1136/bmj.39136.682083.AE |url=http://bmj.com/cgi/pmidlookup?view=long&pmid=17403713 |issn=}}</ref> <ref name="pmid12759327">{{cite journal |author=Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C |title=Composite outcomes in randomized trials: greater precision but with greater uncertainty? |journal=JAMA |volume=289 |issue=19 |pages=2554–9 |year=2003 |month=May |pmid=12759327 |doi=10.1001/jama.289.19.2554 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=12759327 |issn=}}</ref> <ref name="pmid18981486">{{cite journal |author=Lim E, Brown A, Helmy A, Mussa S, Altman DG |title=Composite outcomes in cardiovascular research: a survey of randomized trials |journal=Ann. Intern. Med. |volume=149 |issue=9 |pages=612–7 |year=2008 |month=November |pmid=18981486 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=long&pmid=18981486 |issn=}}</ref> | |||
===Overdiagnosis=== | |||
{{main|Overdiagnosis}} | |||
In trials of [[mass screening]], overdiagnosis is the diagnosis of non-harmful disease. <ref name="pmid16757699">{{cite journal |author=Marcus PM, Bergstralh EJ, Zweig MH, Harris A, Offord KP, Fontana RS |title=Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis |journal=J. Natl. Cancer Inst. |volume=98 |issue=11 |pages=748–56 |year=2006 |month=June |pmid=16757699 |doi=10.1093/jnci/djj207 |url=http://jnci.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=16757699 |issn=}}</ref> Overdiagnosis inflates the importance of the screening problem. | |||
==Analysis== | |||
{{main|Statistics|Statistical significance}} | |||
When protocols for trials are publicly accessible, discrepancies between planned and actual analyses may be found.<ref name="pmid19056791">{{cite journal |author=Chan AW, Hróbjartsson A, Jørgensen KJ, Gøtzsche PC, Altman DG |title=Discrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocols |journal=BMJ |volume=337 |issue= |pages=a2299 |year=2008 |pmid=19056791 |doi= |url= |issn=}}</ref> | |||
Dichotomous outcomes may be summarized with [[relative risk reduction]], [[absolute risk reduction]], or the [[number needed to treat]]. | |||
===Subgroup analyses=== | |||
Subgroup analyses can be misleading due to failure to prespecify hypotheses and to account for multiple comparisons.<ref name="pmid18032770">{{cite journal |author=Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM |title=Statistics in medicine--reporting of subgroup analyses in clinical trials |journal=N. Engl. J. Med. |volume=357 |issue=21 |pages=2189–94 |year=2007 |pmid=18032770 |doi=10.1056/NEJMsr077003}}</ref><ref name="pmid15885299">{{cite journal |author=Schulz KF, Grimes DA |title=Multiplicity in randomised trials II: subgroup and interim analyses |journal=Lancet |volume=365 |issue=9471 |pages=1657–61 |year=2005 |pmid=15885299 |doi=10.1016/S0140-6736(05)66516-6 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(05)66516-6 |issn=}}</ref><ref name="pmid2046134">{{cite journal |author=Yusuf S, Wittes J, Probstfield J, Tyroler HA |title=Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials |journal=JAMA |volume=266 |issue=1 |pages=93–8 |year=1991 |pmid=2046134 |doi=}}</ref> | |||
==Assessing the quality of a trial== | |||
===Cochrane bias scale=== | |||
The Cochrane Collaboration uses a six item tool.<ref>Higgins JPT, Green S (editors). [http://www.mrc-bsu.cam.ac.uk/cochrane/handbook/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm Table 8.5.a: The Cochrane Collaboration's Tool for assessing risk of bias]. [http://www.cochrane-handbook.org/ Cochrane Handbook for Systematic Reviews of Interventions] Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from [http://www.cochrane-handbook.org/ www.cochrane-handbook.org].</ref> | |||
===Jadad score=== | |||
The Jadad score may be used to assess quality and contains three items:<ref name="pmid8721797">{{cite journal |author=Jadad AR, Moore RA, Carroll D, ''et al'' |title=Assessing the quality of reports of randomized clinical trials: is blinding necessary? |journal=Control Clin Trials |volume=17 |issue=1 |pages=1–12 |year=1996 |pmid=8721797 |doi=10.1016/0197-2456(95)00134-4}}</ref> | |||
# Was the study described as randomized (this includes the use of words such as randomly, random, and randomization)? | |||
# Was the study described as double blind? | |||
# Was there a description of withdrawals and dropouts? | |||
Each question is scored one point for a yes answer. In addition, for questions and 2, a point is added if the method was appropriate and a point is deducted if the method is not appropriate (e.g. not effectively randomized or not effectively double-blinded). | |||
==Publication bias== | |||
{{main|Publication bias}} | |||
[[Publication bias]] refers to the tendency that trials that show a positive significant effect are more likely to be published than those that show no effect or are inconclusive. | |||
===Trial registration=== | |||
At the same time, in September 2004, the International Committee of Medical Journal Editors (ICMJE) announced that all trials starting enrollment after July 1, 2005 must be registered prior to consideration for publication in one of the 12 member journals of the Committee.<ref name="pmid15356289">{{cite journal |author=De Angelis C, Drazen JM, Frizelle FA, ''et al'' |title=Clinical trial registration: a statement from the International Committee of Medical Journal Editors |journal=The New England journal of medicine |volume=351 |issue=12 |pages=1250–1 |year=2004 |month=September |pmid=15356289 |doi=10.1056/NEJMe048225 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=15356289&promo=ONFLNS19 |issn=}}</ref> This move was to reduce the risk of publication bias as negative trials that are unpublished would be more easily discoverable. | |||
Available trial registries include: | |||
* http://clinicaltrials.gov | |||
* [[World Health Organization]]'s International Clinical Trial Registry Platform ([http://www.who.int/ictrp/ ICTRP]) | |||
==External validation== | |||
Judging external validity is more difficult than judging internal validity. "External validity refers to the question whether results are generalizable to persons other than the population in the original study."<ref name="10.1093/ije/dyp174">{{Cite journal | doi = 10.1093/ije/dyp174 | pages = dyp174 | |||
| last = Dekkers | first = O M | coauthors = E von Elm, A Algra, J A Romijn, J P Vandenbroucke | |||
| title = How to assess the external validity of therapeutic trials: a conceptual approach | |||
| journal = Int. J. Epidemiol. | accessdate = 2009-04-18 | date = 2009-04-17 | url = http://ije.oxfordjournals.org/cgi/content/abstract/dyp174v1 }}</ref> A framework for assessing external validity proposes:<ref name="10.1093/ije/dyp174"/> | |||
# "The study population might not be representative for the eligibility criteria that were intended. It should be addressed whether the study population differs from the intended source population with respect to characteristics that influence outcome." | |||
# "The target population will, by definition, differ from the study population with respect to geographical, temporal and ethnical conditions. Pondering external validity means asking the question whether these differences may influence study results." | |||
# "It should be assessed whether the study's conclusions can be generalized to target populations that do not meet all the eligibility criteria." | |||
==References== | |||
<references/> | |||
==External links== | |||
* [http://grants2.nih.gov/grants/policy/hs/ U.S. National Institutes of Health: Research Involving Human Subjects] | |||
Revision as of 11:50, 12 May 2009
Template:TOC-right "A clinical trial is defined as a prospective scientific experiment that involves human subjects in whom treatment is initiated for the evaluation of a therapeutic intervention. In a randomized controlled clinical trial, each patient is assigned to receive a specific treatment intervention by a chance mechanism."[1] The theory behind these trials is that the value of a treatment will be shown in an objective way, and, though usually unstated, there is an assumption that the results of the trial will be applicable to the care of patients who have the condition that was treated.
Trials of potential treatments, for ethical reasons, tend to involve multiple stages, starting with small safety tests of the drug or other therapy. Once there is evidence of safety, and preliminary clinical trials, the effort moves to a larger scale: large multicentre clinical trials that are randomised, controlled, and double-blind using a control group of patients (i.e., "arm" of the trial) and an experimental arm.
Trials should be large, so that serious adverse events might be detected even when they occur rarely. Multi-centre trials minimise problems that can arise when a single geographical locus has a population that is not fully representative of the global population, and they can minimise the effect of geographical variations in environment and health care delivery. Randomisation (if the study population is large enough) should mean that the study groups are unbiased. A double-blind trial is one in which neither the patient nor the deliverer of the treatment is aware of the nature of the treatment offered to any particular individual, and this avoids bias caused by the expectations of either the doctor or the patient.
Ethical trials exist in a framework. Before any work with human subjects begins, an appropriate independent review board evaluates the proposed experiment, the risks and benefits to subjects, and way in which informed consent will be given to participation. During the trial, an external safety board monitors progress; it has access to the actual, not double-blinded, data. If the experimental arm of the treatment, in the judgment of the board, is significantly more dangerous than existing therapy, the board can stop the trial. If the experimental treatment is strikingly better than the control arm,
Major developments in randomized controlled trials
In 1996, the Consort Statement was published which improved how trials are reported.[2] The statement was revised in 2001.[3] As of 2005, among high impact journals, "CONSORT was mentioned in the instructions of 36 (22%) journals (see bmj.com), more often in general and internal medicine journals (8/15; 53%) than in specialty journals (28/152; 18%)".[4]
In 2004, the International Committee of Medical Journal Editors (ICMJE) announced that all trials starting enrollment after July 1, 2005 must be registered prior to consideration for publication in one of the 12 member journals of the Committee.[5]
Again, clinical trials exist in a framework. When dealing with treatments, adverse effects may be discovered only after the end of the last phase, or in the larger populations to which a drug, approved for general release based on trial data, is used. New Drug Application is intended to detect hazards that the trials are not powerful enough to find.
Control group
"Control", according to the current Declaration of Helsinki ethical guides, may or may not involve a placebo. If there is no accepted treatment, or the disease is mild and self-limiting, placebo controls may be ethical. If there is accepted treatment, the best accepted treatment becomes the control arm. Trials that are controlled, but not by a placebo, still generate arguments about information value versus ethics.
Placebo controls are important, because the placebo effect can often be strong. The more value a subject believes an unknown drug has, but more placebo effect is has.[6] The use of historical rather than concurrent controls may lead to exagerated estimated of effect.[7]
Placebo effect can be seen in controlled trials of surgical interventions with the control group received a sham procedure.[8][9][10]
The Hawthorne effect is the improvements seen in control subjects simply from participating in research.[11]
Variations in design
Cluster-randomized trials
In some settings, health care providers, or healthcare institutions should be randomized rather than randomizing the research subjects.[12] This should occur when the intervention targets the provider or institutions and thus the results from each subject are not truly independent, but will cluster within the health care provider or healthcare institution. Guidelines exist for conducting cluster randomised trials.[13] Cluster-randomized trials are not always correctly designed and executed.[14]
Designing an adequately sized cluster-randomized trial is based on several factors. One factor is the intraclass (intracluster) correlation coefficient (ICC).[15][16] The ICC between clusters in analogous to the variance between subject in a randomized controlled trial. Just as in Student's t-test for randomized controlled trial more variance between subjects means a larger study is needed, the less correlation between clusters means more clusters are needed.
Before-after studies
Uncontrolled before-after studies and controlled before-after studies probably should not be considered variations of a randomized controlled trial, yet if carefully done offer advantages to observational studies.[17] As in a true cluster-randomized trial, the intervention group can be randomly assigned; however, unlike a cluster-randomized trial, the before-after study does not have enough clusters or groups. An interrupted time series analysis can try to improve plausibility of causation; however, interrupted time series are commonly performed incorrectly.[18]
Crossover trial
In crossover trials, patients start in intervention and controls, but later all patients switch groups.[19]
| Intervention A | |||
|---|---|---|---|
| Given | Not given | ||
| Intervention B | Given | Group 1 | Group 2 |
| Not given | Group 3 | Group 4 | |
Variations on the standard AB, BA design have been proposed.[20][21][22][23]
Factorial design
A factorial design allows two interventions to be be studied with ability to measure the treatment effect of each intervention in isolation and in combination.[24]
n of 1 trial
In a "n of 1" trial, also called single-subject randomized trials, a single patient randomly proceeds through multiple blinded crossover comparisons. This address the concerns that traditional randomized controlled trials may not generalize to a specific patient.[25]
Underlining the difficulty in extrapolating from large trials to individual patients, Sackett proposed the use of N of 1 randomized controlled trials. In these, the patient is both the treatment group and the placebo group, but at different times. Blinding must be done with the collaboration of the pharmacist, and treatment effects must appear and disappear quickly following introduction and cessation of the therapy. This type of trial can be performed for many chronic, stable conditions.[26] The individualized nature of the single-subject randomized trial, and the fact that it often requires the active participation of the patient (questionnaires, diaries), appeals to the patient and promotes better insight and self-management[27][28] as well as patient safety,[25] in a cost-effective manner.
Noninferiority and equivalence randomized trials
As stated in The Declaration of Helsinki by the World Medical Association it is unethical to give any patient a placebo treatment if an existing treatment option is known to be beneficial.[29][30] Many scientists and ethicists consider that the U.S. Food and Drug Administration, by demanding placebo-controlled trials, encourages the systematic violation of the Declaration of Helsinki.[31] In addition, the use of placebo controls remains a convenient way to avoid direct comparisons with a competing drug.
The appropriate use of placebo is being revised.[32][33] When guidelines suggest a placebo is an unethical control, then an "active-control noninferiority trial" may be used.[34] To establish non-inferiority, the following three conditions should be - but frequently are not - established:[34]
- "The treatment under consideration exhibits therapeutic noninferiority to the active control."
- "The treatment would exhibit therapeutic efficacy in a placebo-controlled trial if such a trial were to be performed."
- "The treatment offers ancillary advantages in safety, tolerability, cost, or convenience."
Noninferiority and equivalence randomized trial are difficult to execute well.[34] Guidelines exists for noninferiority and equivalence randomized trials.[35]
Add-on design
"Sometimes a new agent can be assessed by using an 'add-on' study design in which all patients are given standard therapy and are randomly assigned to also receive either new agent or placebo."[32]
Pragmatic trials
Consort has described pragmatic trials and standards for their reporting.[36] CONSORT describes a pragmatic trial as addressing "does the intervention work when used in normal practice" as opposed to an "explanatory trial" that addresses "can the intervention work." CONSORT states the there is not a dichotomy of trial designs, but that pragmatic reflects an attitude in the design. CONSORT offers an example of a pragmatic trial[37] and an explanatory trial.[38]
Ethical issues
The Declaration of Helsinki requires informed consent for participation in a trial. In the United States, there is an approval procedure for clinical trials in human subjects, whether for research only or for potential approval of a commercial drug. Most industrialized countries have such procedures; some permit reciprocal approvals.
Ethics of randomizing subjects
The appropriate use of placebo is being revised.[32][33][39] One tension is the balance between using placebo to increase scientific rigor versus the unnessessary deprival of active treatment to patients. This is the conundrum faced by Martin Arrowsmith in the book by the same name.[40][41]
Comparing a new intervention to a placebo control may not be ethical when an accepted, effective treatment exists. In this case, the new intervention should be compared to the active control to establish whether the standard of care should change.[42] The observation that industry sponsored research may be more likely to conduct trials that have positive results suggest that industry is not picking the most appropriate comparison group.[43] However, it is possible that industry is better at predicting which new innovations are likely to be successful and discontinuing research for less promising interventions before the trial stage.
There are times when placebo control is appropriate even when there is accepted, effective treatment.[32][33][39]
There are ethical concerns in comparing a surgical intervention to sham surgery; however, this has been done.[44][10] Guidelines by the American Medical Association address the use of placebo surgery.[45]
Interim analysis - stopping trials early
Trials are increasingly stopped early[46]; however, this may induce a bias. Data safety and monitoring boards that are independent of the trial are commissioned to conduct interim analyses and make decisions about stopping trials early.[47] [48]
Reasons to stop a trial early are efficacy, safety, and futility.[49][50]
Regarding efficacy, various rules exist that adjust alpha to decide when to stop a trial early.[51][52][53][54][55] A commonly recommended rules are the O'Brien-Fleming (the O'Brien-Fleming rule requires a varying p-value depending on the number of interim analyses) and the Haybittle-Peto (the Haybittle-Peto which requires p<0.001 to stop a trial early) rule.[51][52][56]
Using a more conservative stopping rule reduces the chance of a statistical alpha (Type I) error; however, these rules do not alter that the effect size may be exaggerated.[57][52] According to Bassler, "the more stringent the P-value threshold results must cross to justify stopping the trial, the more likely it is that a trial stopped early will overestimate the treatment effect."[50] A review of trials stopped early found that the earlier a trial was stopped the larger was its reported treatment effect.[46] Accordingly, examples exists of trials whose interim analyses were significant, but the trial was continued and the final analysis was less significant or was insignificant.[58][59][60] Methods to correct for exaggeration exists.[61][57]
As an alternative to the alpha rules, conditional power can help decide when to stop trials early.[62][63]
Seeding trials
Seeding trials are studies sponsored by industry whose "apparent purpose is to test a hypothesis. The true purpose is to get physicians in the habit of prescribing a new drug."[64]
Measuring outcomes
Subjectively assessed outcomes are more susceptible to bias in trials with inadequate allocation concealment.[65]
Missing data
Missing data created decisions regarding what outcome to assign to that patient and what experimental group to assign the patient to.
- Regarding assigning an outcome to the patient, using a 'last observation carried forward' (LOCF) analysis may introduce biases.[66]
- Regarding group assignment, a 'per protocol' analysis may introduce bias compared to an 'intention to treat' analysis.
Surrogate outcomes
The costs and efforts required to measure primary endpoints such as morbidity and mortality make using surrogate outcomes an option. An example is in the treatment of osteoporosis, the primary outcomes are fractures and mortality whereas the surrogate outcome is changes in bone mineral density.[67][68] Other examples of surrogate outcomes are tumor shrinkage or changes in cholesterol level, blood pressure, HbA1c, CD4 cell count.[69] Surrogate markers might be acceptable when "the surrogate must be a correlate of the true clinical outcome and fully capture the net effect of treatment on the clinical outcome".[69]
Composite outcomes
Composite outcomes are problematic if the components of the composite vary in their magnitude and contribution to the composite.[70] [71] [72]
Overdiagnosis
In trials of mass screening, overdiagnosis is the diagnosis of non-harmful disease. [73] Overdiagnosis inflates the importance of the screening problem.
Analysis
When protocols for trials are publicly accessible, discrepancies between planned and actual analyses may be found.[74]
Dichotomous outcomes may be summarized with relative risk reduction, absolute risk reduction, or the number needed to treat.
Subgroup analyses
Subgroup analyses can be misleading due to failure to prespecify hypotheses and to account for multiple comparisons.[75][52][76]
Assessing the quality of a trial
Cochrane bias scale
The Cochrane Collaboration uses a six item tool.[77]
Jadad score
The Jadad score may be used to assess quality and contains three items:[78]
- Was the study described as randomized (this includes the use of words such as randomly, random, and randomization)?
- Was the study described as double blind?
- Was there a description of withdrawals and dropouts?
Each question is scored one point for a yes answer. In addition, for questions and 2, a point is added if the method was appropriate and a point is deducted if the method is not appropriate (e.g. not effectively randomized or not effectively double-blinded).
Publication bias
Publication bias refers to the tendency that trials that show a positive significant effect are more likely to be published than those that show no effect or are inconclusive.
Trial registration
At the same time, in September 2004, the International Committee of Medical Journal Editors (ICMJE) announced that all trials starting enrollment after July 1, 2005 must be registered prior to consideration for publication in one of the 12 member journals of the Committee.[5] This move was to reduce the risk of publication bias as negative trials that are unpublished would be more easily discoverable.
Available trial registries include:
- http://clinicaltrials.gov
- World Health Organization's International Clinical Trial Registry Platform (ICTRP)
External validation
Judging external validity is more difficult than judging internal validity. "External validity refers to the question whether results are generalizable to persons other than the population in the original study."[79] A framework for assessing external validity proposes:[79]
- "The study population might not be representative for the eligibility criteria that were intended. It should be addressed whether the study population differs from the intended source population with respect to characteristics that influence outcome."
- "The target population will, by definition, differ from the study population with respect to geographical, temporal and ethnical conditions. Pondering external validity means asking the question whether these differences may influence study results."
- "It should be assessed whether the study's conclusions can be generalized to target populations that do not meet all the eligibility criteria."
References
- ↑ Stanley K (2007). "Design of randomized controlled trials". Circulation 115 (9): 1164–9. DOI:10.1161/CIRCULATIONAHA.105.594945. PMID 17339574. Research Blogging.
- ↑ Begg C, Cho M, Eastwood S, et al (August 1996). "Improving the quality of reporting of randomized controlled trials. The CONSORT statement". JAMA 276 (8): 637–9. PMID 8773637. [e]
- ↑ Moher D, Schulz KF, Altman D (April 2001). "The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials". JAMA 285 (15): 1987–91. PMID 11308435. [e]
- ↑ Altman DG (May 2005). "Endorsement of the CONSORT statement by high impact medical journals: survey of instructions for authors". BMJ 330 (7499): 1056–7. DOI:10.1136/bmj.330.7499.1056. PMID 15879389. PMC 557224. Research Blogging.
- ↑ 5.0 5.1 De Angelis C, Drazen JM, Frizelle FA, et al (September 2004). "Clinical trial registration: a statement from the International Committee of Medical Journal Editors". The New England journal of medicine 351 (12): 1250–1. DOI:10.1056/NEJMe048225. PMID 15356289. Research Blogging.
- ↑ Waber, Rebecca L., Baba Shiv, Ziv Carmon, and Dan Ariely. 2008. Commercial Features of Placebo and Therapeutic Efficacy. JAMA 299, no. 9:1016-1017.
- ↑ Sacks H, Chalmers TC, Smith H (February 1982). "Randomized versus historical controls for clinical trials". Am. J. Med. 72 (2): 233–40. PMID 7058834. [e]
- ↑ Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA (May 1959). "An evaluation of internal-mammary-artery ligation by a double-blind technic". N. Engl. J. Med. 260 (22): 1115–8. PMID 13657350. [e]
- ↑ Dimond EG, Kittle CF, Crockett JE (April 1960). "Comparison of internal mammary artery ligation and sham operation for angina pectoris". Am. J. Cardiol. 5: 483–6. PMID 13816818. [e]
- ↑ 10.0 10.1 Moseley JB, O'Malley K, Petersen NJ, et al (July 2002). "A controlled trial of arthroscopic surgery for osteoarthritis of the knee". N. Engl. J. Med. 347 (2): 81–8. DOI:10.1056/NEJMoa013259. PMID 12110735. Research Blogging.
Cite error: Invalid
<ref>tag; name "pmid12110735" defined multiple times with different content - ↑ McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P (2007). "The Hawthorne Effect: a randomised, controlled trial". BMC Med Res Methodol 7: 30. DOI:10.1186/1471-2288-7-30. PMID 17608932. PMC 1936999. Research Blogging.
- ↑ Wears RL (2002). "Advanced statistics: statistical methods for analyzing cluster and cluster-randomized data". Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 9 (4): 330–41. PMID 11927463. [e]
- ↑ Campbell MK, Elbourne DR, Altman DG (2004). "CONSORT statement: extension to cluster randomised trials". BMJ 328 (7441): 702–8. DOI:10.1136/bmj.328.7441.702. PMID 15031246. Research Blogging.
- ↑ Eldridge, S., Ashby, D., Bennett, C., Wakelin, M., & Feder, G. (2008). Internal and external validity of cluster randomised trials: systematic review of recent trials. BMJ, http://www.bmj.com/cgi/content/full/bmj.39517.495764.25v1 DOI:10.1136/bmj.39517.495764.25 10.1136/bmj.39517.495764.25.
- ↑ Campbell MK, Fayers PM, Grimshaw JM (2005). "Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research". Clin Trials 2 (2): 99–107. PMID 16279131. [e]
- ↑ Campbell M, Grimshaw J, Steen N (2000). "Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action)". J Health Serv Res Policy 5 (1): 12–6. PMID 10787581. [e]
- ↑ Wyatt JC, Wyatt SM (2003). "When and how to evaluate health information systems?". Int J Med Inform 69 (2-3): 251–9. DOI:10.1016/S1386-5056(02)00108-9. PMID 12810128. Research Blogging.
- ↑ Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE (2003). "Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies". Int J Technol Assess Health Care 19 (4): 613–23. PMID 15095767. [e]
- ↑ Sibbald B, Roberts C (1998). "Understanding controlled trials. Crossover trials". BMJ 316 (7146): 1719. PMID 9614025. [e]
- ↑ Laska E, Meisner M, Kushner HB (December 1983). "Optimal crossover designs in the presence of carryover effects". Biometrics 39 (4): 1087–91. PMID 6671121. [e]
- ↑ Boon PC, Roes KC (March 1999). "Design and analysis issues for crossover designs in phase I clinical studies". J Biopharm Stat 9 (1): 109–28. PMID 10091913. [e]
- ↑ Ebbutt AF (March 1984). "Three-period crossover designs for two treatments". Biometrics 40 (1): 219–24. PMID 6733230. [e]
- ↑ Matthews JN (December 1994). "Multi-period crossover trials". Stat Methods Med Res 3 (4): 383–405. PMID 7889228. [e]
- ↑ Stampfer MJ, Buring JE, Willett W, Rosner B, Eberlein K, Hennekens CH (1985). "The 2 x 2 factorial design: its application to a randomized trial of aspirin and carotene in U.S. physicians". Stat Med 4 (2): 111–6. DOI:10.1002/sim.4780040202. PMID 4023472. Research Blogging.
- ↑ 25.0 25.1 Mahon J, Laupacis A, Donner A, Wood T (1996). "Randomised study of n of 1 trials versus standard practice". BMJ 312 (7038): 1069–74. PMID 8616414. [e]
Cite error: Invalid
<ref>tag; name "pmid8616414" defined multiple times with different content - ↑ Guyatt G et al (1988). "A clinician's guide for conducting randomized trials in individual patients". CMAJ 139: 497–503. PMID 3409138. [e]
- ↑ Brookes ST et al. (2007). ""Me's me and you's you": Exploring patients' perspectives of single patient (n-of-1) trials in the UK". Trials 8: 10. DOI:10.1186/1745-6215-8-10. PMID 17371593. Research Blogging.
- ↑ Langer JC et al. (1993). "The single-subject randomized trial. A useful clinical tool for assessing therapeutic efficacy in pediatric practice". Clinical Pediatrics 32: 654–7. PMID 8299295. [e]
- ↑ World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Retrieved on 2007-11-17.
- ↑ (1997) "World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects". JAMA 277: 925–6. PMID 9062334. [e]
- ↑ Michels KB, Rothman KJ (2003). "Update on unethical use of placebos in randomised trials". Bioethics 17: 188–204. PMID 12812185. [e]
- ↑ 32.0 32.1 32.2 32.3 Temple R, Ellenberg SS (2000). "Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues". Ann Intern Med 133: 455–63. PMID 10975964. [e]
- ↑ 33.0 33.1 33.2 Ellenberg SS, Temple R (2000). "Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: practical issues and specific cases". Ann Intern Med 133: 464–70. PMID 10975965. [e]
- ↑ 34.0 34.1 34.2 Kaul S, Diamond GA (2006). "Good enough: a primer on the analysis and interpretation of noninferiority trials". Ann Intern Med 145: 62–9. PMID 16818930. [e]
Cite error: Invalid
<ref>tag; name "pmid16818930" defined multiple times with different content - ↑ Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ (2006). "Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement". JAMA 295 (10): 1152–60. DOI:10.1001/jama.295.10.1152. PMID 16522836. Research Blogging.
- ↑ Zwarenstein M, Treweek S, Gagnier JJ, et al (2008). "Improving the reporting of pragmatic trials: an extension of the CONSORT statement". BMJ 337: a2390. PMID 19001484. [e]
- ↑ Thomas KJ, MacPherson H, Thorpe L, et al (September 2006). "Randomised controlled trial of a short course of traditional acupuncture compared with usual care for persistent non-specific low back pain". BMJ 333 (7569): 623. DOI:10.1136/bmj.38878.907361.7C. PMID 16980316. PMC 1570824. Research Blogging.
- ↑ (August 1991) "Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators". N. Engl. J. Med. 325 (7): 445–53. PMID 1852179. [e]
- ↑ 39.0 39.1 Emanuel EJ, Miller FG (2001). "The ethics of placebo-controlled trials--a middle ground". N. Engl. J. Med. 345 (12): 915–9. PMID 11565527. [e]
- ↑ Hausler, Thomas (2008-05-01). "In retrospect: When business became biology's plague". Nature 453 (7191): 38. DOI:10.1038/453038a. ISSN 0028-0836. Retrieved on 2008-11-12. Research Blogging.
- ↑ Pastor, John (2008-06-26). "The ethical basis of the null hypothesis". Nature 453 (7199): 1177. DOI:10.1038/4531177b. ISSN 0028-0836. Retrieved on 2008-11-12. Research Blogging.
- ↑ Rothman KJ, Michels KB (1994). "The continuing unethical use of placebo controls". N. Engl. J. Med. 331 (6): 394–8. PMID 8028622. [e]
- ↑ Djulbegovic B, Lacevic M, Cantor A, et al (2000). "The uncertainty principle and industry-sponsored research". Lancet 356 (9230): 635–8. PMID 10968436. [e]
- ↑ Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA: An evaluation of internal-mammary-artery ligation by a double-blind technique. N Engl J Med 1959;260:1115-1118.
- ↑ Tenery R, Rakatansky H, Riddick FA, et al (2002). "Surgical "placebo" controls". Ann. Surg. 235 (2): 303–7. PMID 11807373. [e]
- ↑ 46.0 46.1 Montori VM, Devereaux PJ, Adhikari NK, et al (November 2005). "Randomized trials stopped early for benefit: a systematic review". JAMA 294 (17): 2203–9. DOI:10.1001/jama.294.17.2203. PMID 16264162. Research Blogging.
- ↑ Trotta, F., G. Apolone, S. Garattini, and G. Tafuri. 2008. Stopping a trial early in oncology: for patients or for industry? Ann Oncol mdn042. http://dx.doi.org/10.1093/annonc/mdn042
- ↑ Slutsky AS, Lavery JV (March 2004). "Data Safety and Monitoring Boards". N. Engl. J. Med. 350 (11): 1143–7. DOI:10.1056/NEJMsb033476. PMID 15014189. Research Blogging.
- ↑ Borer JS, Gordon DJ, Geller NL (April 2008). "When should data and safety monitoring committees share interim results in cardiovascular trials?". JAMA 299 (14): 1710–2. DOI:10.1001/jama.299.14.1710. PMID 18398083. Research Blogging.
- ↑ 50.0 50.1 Bassler D, Montori VM, Briel M, Glasziou P, Guyatt G (March 2008). "Early stopping of randomized clinical trials for overt efficacy is problematic". J Clin Epidemiol 61 (3): 241–6. DOI:10.1016/j.jclinepi.2007.07.016. PMID 18226746. Research Blogging.
- ↑ 51.0 51.1 Pocock SJ (November 2005). "When (not) to stop a clinical trial for benefit". JAMA 294 (17): 2228–30. DOI:10.1001/jama.294.17.2228. PMID 16264167. Research Blogging.
- ↑ 52.0 52.1 52.2 52.3 Schulz KF, Grimes DA (2005). "Multiplicity in randomised trials II: subgroup and interim analyses". Lancet 365 (9471): 1657–61. DOI:10.1016/S0140-6736(05)66516-6. PMID 15885299. Research Blogging.
- ↑ Grant A (September 2004). "Stopping clinical trials early". BMJ 329 (7465): 525–6. DOI:10.1136/bmj.329.7465.525. PMID 15345605. Research Blogging.
- ↑ O'Brien PC, Fleming TR (September 1979). "A multiple testing procedure for clinical trials". Biometrics 35 (3): 549–56. PMID 497341. [e]
- ↑ Bauer P, Köhne K (December 1994). "Evaluation of experiments with adaptive interim analyses". Biometrics 50 (4): 1029–41. PMID 7786985. [e] This method was used by PMID: 18184958
- ↑ DeMets, David L.; Susan S. Ellenberg; Fleming, Thomas J. (2002). Data Monitoring Committees in Clinical Trials: a Practical Perspective. New York: J. Wiley & Sons. ISBN 0-471-48986-7.
- ↑ 57.0 57.1 Pocock SJ, Hughes MD (December 1989). "Practical problems in interim analyses, with particular regard to estimation". Control Clin Trials 10 (4 Suppl): 209S–221S. PMID 2605969. [e]
- ↑ Pocock S, Wang D, Wilhelmsen L, Hennekens CH (May 2005). "The data monitoring experience in the Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) program". Am. Heart J. 149 (5): 939–43. DOI:10.1016/j.ahj.2004.10.038. PMID 15894981. Research Blogging.
- ↑ Wheatley K, Clayton D (February 2003). "Be skeptical about unexpected large apparent treatment effects: the case of an MRC AML12 randomization". Control Clin Trials 24 (1): 66–70. PMID 12559643. [e]
- ↑ Abraham E, Reinhart K, Opal S, et al (July 2003). "Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial". JAMA 290 (2): 238–47. DOI:10.1001/jama.290.2.238. PMID 12851279. Research Blogging.
- ↑ Hughes MD, Pocock SJ (December 1988). "Stopping rules and estimation problems in clinical trials". Stat Med 7 (12): 1231–42. PMID 3231947. [e]
- ↑ (2005) Statistical Monitoring of Clinical Trials: Fundamentals for Investigators. Berlin: Springer. ISBN 0-387-27781-1.
- ↑ Lachin JM (October 2006). "Operating characteristics of sample size re-estimation with futility stopping based on conditional power". Stat Med 25 (19): 3348–65. DOI:10.1002/sim.2455. PMID 16345019. Research Blogging.
- ↑ Hill KP, Ross JS, Egilman DS, Krumholz HM (August 2008). "The ADVANTAGE seeding trial: a review of internal documents". Ann. Intern. Med. 149 (4): 251–8. PMID 18711155. [e]
- ↑ Wood, Lesley; Matthias Egger, Lise Lotte Gluud, Kenneth F Schulz, Peter Juni, Douglas G Altman, Christian Gluud, Richard M Martin, Anthony J G Wood, Jonathan A C Sterne (2008-03-15). "Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study". BMJ 336 (7644): 601-605. DOI:10.1136/bmj.39465.451748.AD. Retrieved on 2008-03-14. Research Blogging.
- ↑ Hauser WA, Rich SS, Annegers JF, Anderson VE (August 1990). "Seizure recurrence after a 1st unprovoked seizure: an extended follow-up". Neurology 40 (8): 1163–70. PMID 2381523. [e]
- ↑ Li Z, Chines AA, Meredith MP (2004). "Statistical validation of surrogate endpoints: is bone density a valid surrogate for fracture?". J Musculoskelet Neuronal Interact 4 (1): 64–74. DOI:10.1081/BIP-120024209. PMID 15615079. Research Blogging.
- ↑ Li Z, Meredith MP (2003). "Exploring the relationship between surrogates and clinical outcomes: analysis of individual patient data vs. meta-regression on group-level summary statistics". J Biopharm Stat 13 (4): 777–92. DOI:10.1081/BIP-120024209. PMID 14584722. Research Blogging.
- ↑ 69.0 69.1 Fleming TR, DeMets DL (1996). "Surrogate end points in clinical trials: are we being misled?". Ann. Intern. Med. 125 (7): 605–13. PMID 8815760. [e]
- ↑ Ferreira-González I, Busse JW, Heels-Ansdell D, et al (April 2007). "Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials". BMJ 334 (7597): 786. DOI:10.1136/bmj.39136.682083.AE. PMID 17403713. PMC 1852019. Research Blogging.
- ↑ Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C (May 2003). "Composite outcomes in randomized trials: greater precision but with greater uncertainty?". JAMA 289 (19): 2554–9. DOI:10.1001/jama.289.19.2554. PMID 12759327. Research Blogging.
- ↑ Lim E, Brown A, Helmy A, Mussa S, Altman DG (November 2008). "Composite outcomes in cardiovascular research: a survey of randomized trials". Ann. Intern. Med. 149 (9): 612–7. PMID 18981486. [e]
- ↑ Marcus PM, Bergstralh EJ, Zweig MH, Harris A, Offord KP, Fontana RS (June 2006). "Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis". J. Natl. Cancer Inst. 98 (11): 748–56. DOI:10.1093/jnci/djj207. PMID 16757699. Research Blogging.
- ↑ Chan AW, Hróbjartsson A, Jørgensen KJ, Gøtzsche PC, Altman DG (2008). "Discrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocols". BMJ 337: a2299. PMID 19056791. [e]
- ↑ Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM (2007). "Statistics in medicine--reporting of subgroup analyses in clinical trials". N. Engl. J. Med. 357 (21): 2189–94. DOI:10.1056/NEJMsr077003. PMID 18032770. Research Blogging.
- ↑ Yusuf S, Wittes J, Probstfield J, Tyroler HA (1991). "Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials". JAMA 266 (1): 93–8. PMID 2046134. [e]
- ↑ Higgins JPT, Green S (editors). Table 8.5.a: The Cochrane Collaboration's Tool for assessing risk of bias. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
- ↑ Jadad AR, Moore RA, Carroll D, et al (1996). "Assessing the quality of reports of randomized clinical trials: is blinding necessary?". Control Clin Trials 17 (1): 1–12. DOI:10.1016/0197-2456(95)00134-4. PMID 8721797. Research Blogging.
- ↑ 79.0 79.1 Dekkers, O M; E von Elm, A Algra, J A Romijn, J P Vandenbroucke (2009-04-17). "How to assess the external validity of therapeutic trials: a conceptual approach". Int. J. Epidemiol.: dyp174. DOI:10.1093/ije/dyp174. Retrieved on 2009-04-18. Research Blogging.
External links
- Pages with reference errors
- Pages using PMID magic links
- Pages using ISBN magic links
- Subpages
- Health Sciences Extra Subpages
- Health Sciences Approved Extra Subpages
- Citable versions of articles
- Health Sciences Citable Version Subpages
- All Content
- Health Sciences Content
- Pages with too many expensive parser function calls