imported>Robert Badgett |
imported>Robert Badgett |
| (6 intermediate revisions by 2 users not shown) |
| Line 11: |
Line 11: |
| MedlinePlus = | | | MedlinePlus = | |
| }} | | }} |
| | | {{TOC|left}} |
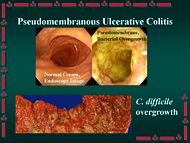
| In [[medicine]], '''pseudomembranous enterocolitis''' is an "acute inflammation of the intestinal mucosa that is characterized by the presence of pseudomembranes or plaques in the small intestine (pseudomembranous enteritis) and the large intestine (pseudomembranous colitis). It is commonly associated with [[antibiotic]] therapy and [[clostridium difficile]] colonization."<ref>{{MeSH}}</ref> | | In [[gastroenterology]], '''pseudomembranous enterocolitis''' is an "acute inflammation of the intestinal mucosa that is characterized by the presence of pseudomembranes or plaques in the small intestine (pseudomembranous enteritis) and the large intestine (pseudomembranous colitis). It is commonly associated with [[antibiotic]] therapy and [[clostridium difficile]] colonization."<ref>{{MeSH}}</ref> The disorder is an increasing matter of concern, as it is one of the more common [[nosocomial]] infections, but is also being seen in community-acquired cases. |
|
| |
|
| ==Epidemiology== | | ==Epidemiology== |
| Line 24: |
Line 24: |
|
| |
|
| ==Treatment== | | ==Treatment== |
| [[Clinical practice guideline]]s are available to direct management.<ref name="pmid20307191">{{cite journal| author=Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC et al.| title=Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). | journal=Infect Control Hosp Epidemiol | year= 2010 | volume= 31 | issue= 5 | pages= 431-55 | pmid=20307191
| | {{Seealso|Clostridium difficile}} |
| | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=20307191 | doi=10.1086/651706 }} </ref>
| |
| | |
| ===Antibiotics===
| |
| Various antibiotics have been studied in [[randomized controlled trial]]s.<ref name="pmid17636768">{{cite journal |author=Nelson R |title=Antibiotic treatment for Clostridium difficile-associated diarrhea in adults |journal=Cochrane Database Syst Rev |volume= |issue=3 |pages=CD004610 |year=2007 |pmid=17636768 |doi=10.1002/14651858.CD004610.pub3 |url=http://dx.doi.org/10.1002/14651858.CD004610.pub3 |issn=}}</ref><ref name="pmid8722937">{{cite journal |author=Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W |title=Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea |journal=Clin. Infect. Dis. |volume=22 |issue=5 |pages=813–8 |year=1996 |month=May |pmid=8722937 |doi= |url= |issn=}}</ref><ref name="pmid19133801">{{cite journal |author=Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF |title=Nitazoxanide versus Vancomycin in Clostridium difficile Infection: A Randomized, Double-Blind Study |journal=Clin. Infect. Dis. |volume= |issue= |pages= |year=2009 |month=January |pmid=19133801 |doi=10.1086/596552 |url=http://www.journals.uchicago.edu/doi/abs/10.1086/596552?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov |issn=}}</ref> [[Teicoplanin]] may be the most effective antibiotic.<ref name="pmid17636768">{{cite journal |author=Nelson R |title=Antibiotic treatment for Clostridium difficile-associated diarrhea in adults |journal=Cochrane Database Syst Rev |volume= |issue=3 |pages=CD004610 |year=2007 |pmid=17636768 |doi=10.1002/14651858.CD004610.pub3 |url=http://dx.doi.org/10.1002/14651858.CD004610.pub3 |issn=}}</ref><ref name="pmid8722937">{{cite journal |author=Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W |title=Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea |journal=Clin. Infect. Dis. |volume=22 |issue=5 |pages=813–8 |year=1996 |month=May |pmid=8722937 |doi= |url= |issn=}}</ref> [[Vancomycin]] has an insignificant trend towards being better than [[metronidazole]];<ref name="pmid17636768">{{cite journal |author=Nelson R |title=Antibiotic treatment for Clostridium difficile-associated diarrhea in adults |journal=Cochrane Database Syst Rev |volume= |issue=3 |pages=CD004610 |year=2007 |pmid=17636768 |doi=10.1002/14651858.CD004610.pub3 |url=http://dx.doi.org/10.1002/14651858.CD004610.pub3 |issn=}}</ref> metronidazole is likely to be the least expensive of the group and is not a last resort for other highly resistant organisms.
| |
| | |
| Consider vancomycin if the patient has two or more points from the following:<ref>Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007 Aug 1;45(3):302-7. PMID 17599306</ref>
| |
| * 1 point for each of
| |
| ** WBC > 15k
| |
| ** albumin < 2.5
| |
| ** age > 60
| |
| ** temperature > 38.3° C
| |
| * 2 points for each of
| |
| ** Pseudomembranous colitis
| |
| ** Intensive care
| |
| | |
| It should be noted that vancomycin is given orally in this condition, and is not absorbed systemically. While it still needs to be used with care to avoid the development of resistant organisms, the risk may be lower than if it is used as a parenteral therapy for already resistant pathogens such as [[Staphylococcus aureus | methicillin-resistant ''Staphylococcus aureus'']]
| |
| | |
| A case serioes suggests [[tigecycline]] for severe disease.<ref name="pmid19435431">{{cite journal |author=Herpers BL, Vlaminckx B, Burkhardt O, ''et al.'' |title=Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection |journal=Clin. Infect. Dis. |volume=48 |issue=12 |pages=1732–5 |year=2009 |month=June |pmid=19435431 |doi=10.1086/599224 |url=http://www.journals.uchicago.edu/doi/abs/10.1086/599224?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov |issn=}}</ref>
| |
| | |
| ===Administration of bacteria===
| |
| [[Probiotic]] administration may<ref name="pmid16635227">{{cite journal |author=McFarland LV |title=Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease |journal=Am. J. Gastroenterol. |volume=101 |issue=4 |pages=812–22 |year=2006 |month=April |pmid=16635227 |doi=10.1111/j.1572-0241.2006.00465.x |url=http://dx.doi.org/10.1111/j.1572-0241.2006.00465.x |issn=}}</ref> or may not <ref name="pmid18254055">{{cite journal |author=Pillai A, Nelson R |title=Probiotics for treatment of Clostridium difficile-associated colitis in adults |journal=Cochrane Database Syst Rev |volume= |issue=1 |pages=CD004611 |year=2008 |pmid=18254055 |doi=10.1002/14651858.CD004611.pub2 |url=http://dx.doi.org/10.1002/14651858.CD004611.pub2 |issn=}}</ref>help according to a [[meta-analysis|meta-analyses]] and a more recent [[randomized controlled trial]].<ref name="pmid18840110">{{cite journal |author=Klarin B, Wullt M, Palmquist I, Molin G, Larsson A, Jeppsson B |title=Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics |journal=Acta Anaesthesiol Scand |volume=52 |issue=8 |pages=1096–102 |year=2008 |month=September |pmid=18840110 |doi=10.1111/j.1399-6576.2008.01748.x |url=http://dx.doi.org/10.1111/j.1399-6576.2008.01748.x |issn=}}</ref> However, probiotics can be harmful among [[intensive care]] patients.<ref name="pmid15889360">{{cite journal |author=Muñoz P, Bouza E, Cuenca-Estrella M, ''et al'' |title=Saccharomyces cerevisiae fungemia: an emerging infectious disease |journal=Clin. Infect. Dis. |volume=40 |issue=11 |pages=1625–34 |year=2005 |month=June |pmid=15889360 |doi=10.1086/429916 |url=http://www.journals.uchicago.edu/cgi-bin/resolve?CID35391 |issn=}}</ref>
| |
| | |
| ===Monoclonal antibodies===
| |
| Adjunct treatment with [[monoclonal antibody|monoclonal antibodies]] may reduce the risk of recurrence from 38% to 7%.<ref name="pmid20089970">{{cite journal| author=Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN et al.| title=Treatment with monoclonal antibodies against Clostridium difficile toxins. | journal=N Engl J Med | year= 2010 | volume= 362 | issue= 3 | pages= 197-205 | pmid=20089970
| |
| | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&email=badgett@uthscdsa.edu&retmode=ref&cmd=prlinks&id=20089970 | doi=10.1056/NEJMoa0907635 }} <!--Formatted by http://sumsearch.uthscsa.edu/cite/--></ref>
| |
| | |
| ===Recurrent infection===
| |
| A [[clinical prediction rule]] found that recurrent infection is more likely if age is more than 65 years, the patient has severe or fulminant illness, and additional antibiotic exposure occurs after after treatment of the initial Clostridium difficile infection.<ref name="pmid19162027">{{cite journal |author=Hu MY, Katchar K, Kyne L, ''et al'' |title=Prospective Derivation and Validation of a Clinical Prediction Rule for Recurrent Clostridium difficile Infection |journal=Gastroenterology |volume= |issue= |pages= |year=2008 |month=December |pmid=19162027 |doi=10.1053/j.gastro.2008.12.038 |url=http://linkinghub.elsevier.com/retrieve/pii/S0016-5085(08)02262-2 |issn=}}</ref> Use of antacids may also be a risk factor for recurrence.<ref name="pmid18951661">{{cite journal |author=Garey KW, Sethi S, Yadav Y, DuPont HL |title=Meta-analysis to assess risk factors for recurrent Clostridium difficile infection |journal=J. Hosp. Infect. |volume=70 |issue=4 |pages=298–304 |year=2008 |month=December |pmid=18951661 |doi=10.1016/j.jhin.2008.08.012 |url=http://linkinghub.elsevier.com/retrieve/pii/S0195-6701(08)00352-6 |issn=}}</ref>
| |
| | |
| Asymptomatic carriage should not be treated according to a [[meta-analysis]].<ref name="pmid9500319">{{cite journal |author=Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN |title=Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea |journal=Lancet |volume=351 |issue=9103 |pages=633–6 |year=1998 |month=February |pmid=9500319 |doi=10.1016/S0140-6736(97)08062-8 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(97)08062-8 |issn=}}</ref>
| |
| | |
| ====Antibiotics====
| |
| Observational [[cohort study|studies]] conflict regarding the best agent and suggest [[vancomycin]] may<ref name="pmid12135033">{{cite journal |author=McFarland LV, Elmer GW, Surawicz CM |title=Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease |journal=Am. J. Gastroenterol. |volume=97 |issue=7 |pages=1769–75 |year=2002 |month=July |pmid=12135033 |doi=10.1016/S0002-9270(02)04195-3 |url= |issn=}}</ref> or may not<ref name="pmid16477549">{{cite journal |author=Pépin J, Routhier S, Gagnon S, Brazeau I |title=Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada |journal=Clin. Infect. Dis. |volume=42 |issue=6 |pages=758–64 |year=2006 |month=March |pmid=16477549 |doi=10.1086/501126 |url=http://www.journals.uchicago.edu/doi/abs/10.1086/501126?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov |issn=}}</ref> be better than [[metronidazole]]. Various methods exist for the administration of [[vancomycin]]<ref name="pmid12135033">{{cite journal |author=McFarland LV, Elmer GW, Surawicz CM |title=Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease |journal=Am. J. Gastroenterol. |volume=97 |issue=7 |pages=1769–75 |year=2002 |month=July |pmid=12135033 |doi=10.1016/S0002-9270(02)04195-3 |url= |issn=}}</ref><ref name="pmid4050760">{{cite journal |author=Tedesco FJ, Gordon D, Fortson WC |title=Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis |journal=Am. J. Gastroenterol. |volume=80 |issue=11 |pages=867–8 |year=1985 |month=November |pmid=4050760 |doi= |url= |issn=}}</ref> and [[metronidazole]]<ref name="pmid19086244">{{cite journal |author=Mattila E, Anttila VJ, Broas M, ''et al'' |title=A randomized, double-blind study comparing Clostridium difficile immune whey and metronidazole for recurrent Clostridium difficile-associated diarrhoea: efficacy and safety data of a prematurely interrupted trial |journal=Scand. J. Infect. Dis. |volume=40 |issue=9 |pages=702–8 |year=2008 |pmid=19086244 |doi= |url= |issn=}}</ref>.
| |
| | |
| {| class="wikitable"
| |
| |+ [[Cohort study|Cohort studies]] and case series of the treatment of Clostridium difficile associated diarrhea
| |
| ! !! Patients !! Intervention / duration!! Comparison !! Outcome:<br/>Recurrence rate
| |
| |-
| |
| |colspan="5" align="center"|Approximately 2 weeks
| |
| |-
| |
| |Vancomycin constant dose<ref name="pmid16477549"/>||171 patients||0.5 to 2 grams daily<br/>10 - 14 days||align="center"|NA||40%
| |
| |-
| |
| |Metronidazole constant dose<ref name="pmid16477549"/>||115 patients||1.0 to 1.5 grams per day<br/>10 - 14 days||align="center"|NA||37%
| |
| |-
| |
| |Metronidazole constant dose<ref name="pmid19086244"/>||20 patients||Metronidzole 1200 mg daily<br/>14 days||align="center"|NA||45%
| |
| |-
| |
| | Vancomycin constant dose<ref name="pmid12135033"/>||83 patients||0.5 to 3 grams/day<br/>10 - 16 days||align="center"|NA||54%
| |
| |-
| |
| | high dose<ref name="pmid12135033"/>||21 patients|| ≥2 grams/day<br/>10 - 16 days||align="center"|NA|| 43%
| |
| |-
| |
| | medium dose<ref name="pmid12135033"/>||14 patients|| 1 - 2 grams/day<br/>10 - 16 days||align="center"|NA|| 71%
| |
| |-
| |
| | low dose<ref name="pmid12135033"/>||48 patients|| < 1 grams/day<br/>10 - 16 days||align="center"|NA|| 54%
| |
| |-
| |
| | Metronidazole constant dose<ref name="pmid12135033"/>||36 patients||0.5 to 3 grams/day<br/>10 - 16 days||align="center"|NA||42%
| |
| |-
| |
| |colspan="5" align="center"|Approximately 3 weeks
| |
| |-
| |
| |Vancomycin tapered dose<ref name="pmid12135033"/>||29 patients||Varying doses<br/>21.5 ± 10.0 days||align="center"|NA||31%
| |
| |-
| |
| |Vancomycin pulsed dose<ref name="pmid12135033"/>||7 patients||Varying doses<br/>21 days||align="center"|NA||14%
| |
| |-
| |
| |colspan="5" align="center"|More than 3 weeks
| |
| |-
| |
| |Vancomycin followed by rifaximin<ref name="pmid17304459"/>||8 patients||Vancomycin of unknown duration followed by rifaximin 400–800 mg daily for 14 days<br/>≥ 21 days||align="center"|NA||13%
| |
| |-
| |
| |Vancomycin tapered & pulsed dose<ref name="pmid4050760"/>||22 patients||125 mg four times daily tapered to 125 mg every third day<br/>42 days||align="center"|NA||0%
| |
| |}
| |
| | |
| Serial therapy with [[vancomycin]] and [[rifaximin]] has been studied in a small uncontrolled series of patients.<ref name="pmid17304459">{{cite journal |author=Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN |title=Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin |journal=Clin. Infect. Dis. |volume=44 |issue=6 |pages=846–8 |year=2007 |month=March |pmid=17304459 |doi=10.1086/511870 |url=http://www.journals.uchicago.edu/doi/abs/10.1086/511870?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov |issn=}}</ref>
| |
| | |
| ====Administration of bacteria====
| |
| [[Probiotic]]s may help.<ref name="pmid18545161">{{cite journal |author=Surawicz CM |title=Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea |journal=J. Clin. Gastroenterol. |volume=42 Suppl 2 |issue= |pages=S64–70 |year=2008 |month=July |pmid=18545161 |doi=10.1097/MCG.0b013e3181646d09 |url= |issn=}}</ref> However, probiotics can be harmful among [[intensive care]] patients.<ref name="pmid15889360">{{cite journal |author=Muñoz P, Bouza E, Cuenca-Estrella M, ''et al'' |title=Saccharomyces cerevisiae fungemia: an emerging infectious disease |journal=Clin. Infect. Dis. |volume=40 |issue=11 |pages=1625–34 |year=2005 |month=June |pmid=15889360 |doi=10.1086/429916 |url=http://www.journals.uchicago.edu/cgi-bin/resolve?CID35391 |issn=}}</ref>
| |
| | |
| Rectal infusion of feces helped according to case reports.<ref name="pmid6137662">{{cite journal |author=Schwan A, Sjölin S, Trottestam U, Aronsson B |title=Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces |journal=Lancet |volume=2 |issue=8354 |pages=845 |year=1983 |month=October |pmid=6137662 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(83)90753-5 |issn=}}</ref><ref name="pmid18808083">{{cite journal |author=Nieuwdorp M, van Nood E, Speelman P, ''et al'' |title=[Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] |language=Dutch; Flemish |journal=Ned Tijdschr Geneeskd |volume=152 |issue=35 |pages=1927–32 |year=2008 |month=August |pmid=18808083 |doi= |url= |issn=}}</ref>
| |
|
| |
|
| ==References== | | ==References== |
| <references/> | | <references/> |