Penicillin V: Difference between revisions
imported>David E. Volk (New page: {{subpages}} right|thumb|250px|{{#ifexist:Template:Penicillin V structure.jpg/credit|{{Penicillin V structure.jpg/credit}}<br/>|}}Penicillin V '''Pen...) |
imported>David E. Volk mNo edit summary |
||
| Line 2: | Line 2: | ||
[[Image:Penicillin V structure.jpg|right|thumb|250px|{{#ifexist:Template:Penicillin V structure.jpg/credit|{{Penicillin V structure.jpg/credit}}<br/>|}}Penicillin V]] | [[Image:Penicillin V structure.jpg|right|thumb|250px|{{#ifexist:Template:Penicillin V structure.jpg/credit|{{Penicillin V structure.jpg/credit}}<br/>|}}Penicillin V]] | ||
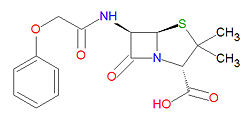
'''Penicillin V''' is a broad-spectrum, beta-[[lactam]]-based [[antibiotic]] used to treat mild to severe infections due to gram-positive bacteria. Is used to treat dental, hear, middle ear, respiratory tract and skin infections, and can also treat [[rheumatic fever|rheumatic] and [[scarlet fever]]s. | '''Penicillin V''' is a broad-spectrum, beta-[[lactam]]-based [[antibiotic]] used to treat mild to severe infections due to gram-positive bacteria. Is used to treat dental, hear, middle ear, respiratory tract and skin infections, and can also treat [[rheumatic fever|rheumatic]] and [[scarlet fever]]s. | ||
== Mechanism of action == | == Mechanism of action == | ||
Revision as of 15:26, 11 February 2008
Penicillin V is a broad-spectrum, beta-lactam-based antibiotic used to treat mild to severe infections due to gram-positive bacteria. Is used to treat dental, hear, middle ear, respiratory tract and skin infections, and can also treat rheumatic and scarlet fevers.
Mechanism of action
Like other penicillin-like drugs, penicillin V works by binding to specific penicillin-binding proteins in bacterial cell walls and blocking the final cross-linking step in the synthesis of bacterial cell walls. This induces autolysis of the bactertial cells by autolysins.
Chemistry
Penicillin V is stable against degradation by beta-lactamases, including penicillinases, and cephalosporinases. Its IUPAC chemical name is (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[[2-(phenoxy)acetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has molecular formula C16H18N2O5S (MW = 350.3895 g/mol).
External links
- Penicillin V - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Drug Bank [1]