Abacavir: Difference between revisions
imported>David E. Volk (Chem infobox) |
imported>David E. Volk mNo edit summary |
||
| Line 4: | Line 4: | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
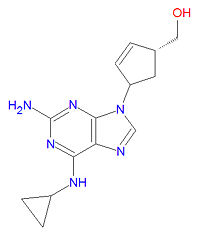
|image=[[Image:Abacavir structure.jpg|right|thumb| | |image=[[Image:Abacavir structure.jpg|right|thumb|200px|{{#ifexist:Template:Abacavir structure.jpg/credit|{{Abacavir structure.jpg/credit}}<br/>|}}Abacavir, an antiviral drug, is a nucleotide analog.]] | ||
|width= | |width=200px | ||
|molname=abacavir | |molname=abacavir | ||
|synonyms= ABC | |synonyms= ABC | ||
Revision as of 08:59, 22 March 2008
|
| |||||||
| abacavir | |||||||
| |||||||
| Uses: | HIV | ||||||
| Properties: | reverse transcriptase inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Abacavir, abbreviated ABC, is a nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV/AIDS. It becomes phosphorylated by cellular enzymes into an active metabolite, caravir triphosphate, which is an analog of deoxyguanosine-5'-triphosphate (dGTP). Caravir triphosphate gets incorporated into viral DNA where it acts as a chain terminator because it does not contain a 3'-hydroxy group needed to link with the next DNA base. It also inhibits HIV-1 transcriptase by binding to the enzyme so that the natural base, dGTP, cannot bind. Because the drugs' metabolism involves the enzyme alcohol dehydrogenase, use of alcohol with this drug should be avoided.
Chemistry
The IUPAC chemical name of abacavir is [(1R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]-1-cyclopent-2-enyl]methanol, and is has chemical formula C14H18N6O, giving it a molecular mass of 286.3323 g/mol. It is a purine analog.
External Links
- Abacavir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank