Antibiotic: Difference between revisions
imported>David E. Volk |
imported>David E. Volk m (→Cephalosporins) |
||
| Line 28: | Line 28: | ||
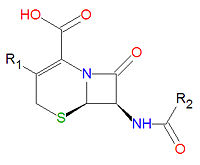
[[Image:Cephalosporin base.jpg|right|thumb|200px|{{#ifexist:Template:Cephalosporin base.jpg/credit|{{Cephalosporin base.jpg/credit}}<br/>|}}Base structure of all cephalosporins.]] | [[Image:Cephalosporin base.jpg|right|thumb|200px|{{#ifexist:Template:Cephalosporin base.jpg/credit|{{Cephalosporin base.jpg/credit}}<br/>|}}Base structure of all cephalosporins.]] | ||
[[Cephalosporins]] are a class of [[antibiotic]] compounds sharing a common base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, [[cephalosporin C]]. [[Penicillin]]s are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a [[lactam|beta-lactam]], which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely related[[cephamycin]]s are collectively referred to as [[cephems]]. In general, second generation and later cephalosporins have a broader spectrum of activity against [[Gram-negative]] bacteria. | [[Cephalosporins]] are a class of [[antibiotic]] compounds sharing a common gamma-[[lactam]] base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, [[cephalosporin C]]. [[Penicillin]]s are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a [[lactam|beta-lactam]], which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely related[[cephamycin]]s are collectively referred to as [[cephems]]. In general, second generation and later cephalosporins have a broader spectrum of activity against [[Gram-negative]] bacteria. | ||
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the [[International Nonproprietary Name]]s (INN) suggested by the [[World Health Organization]] use the "cef" spelling for the generic drug name of all cephalosporins. | Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the [[International Nonproprietary Name]]s (INN) suggested by the [[World Health Organization]] use the "cef" spelling for the generic drug name of all cephalosporins. | ||
Revision as of 14:18, 11 July 2008
Antibiotics are "substances that reduce the growth or reproduction of bacteria."[1] They interfere with the life cycle of bacteria in a number of different ways. Some antibiotics, like penicillin, interfere with cell wall synthesis, while others are reverse transcriptase inhibitors that interefere with the production of viral RNA and DNA. Other antibiotics are nucleoside analogs that get incorporated into the viral RNA or DNA and act a chain terminators.
Misuse
One study on respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who they believed expected them, although they correctly identified only about 1 in 4 of those patients".[2] Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescribing of antibiotics. [3] Delaying antibiotics for 48 hours while observing for spontaneous resolution of respiratory tract infections may reduce antibiotic usage; however, this strategy may reduce patient satisfaction.[4]
Classes of antibiotics
Penicillins
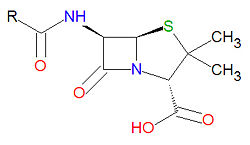
Penicillins have a common beta-lactam base structure, as shown, where R represents different chemical groups. Penicillins work by binding to penicillin-binding proteins irreversibly in a ring-opening reaction and disrupting bacterial cell wall synthesis. Some bacteria are resistant to penicillin because they have acquired the ability to make penicillinases, enzymes which degrade penicillin.
- Amoxicillin
- Ampicillin
- Azlocillin
- Carbenicillin
- Cloxacillin
- Dicloxacillin
- Flucloxacillin
- Hetacillin
- Oxacillin
- Mezlocillin
- Penicillin G
- Penicillin V
- Piperacillin
Cephalosporins
Cephalosporins are a class of antibiotic compounds sharing a common gamma-lactam base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, cephalosporin C. Penicillins are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely relatedcephamycins are collectively referred to as cephems. In general, second generation and later cephalosporins have a broader spectrum of activity against Gram-negative bacteria.
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the International Nonproprietary Names (INN) suggested by the World Health Organization use the "cef" spelling for the generic drug name of all cephalosporins.
|
Aminoglycosides
Tetracyclines
|
Quinalones
Other Antibiotics
- Azithromycin
- Aztreonam
- Chloramphenicol
- Clarithromycin
- Clindamycin
- Clomocycline
- Colistin
- Dirithromycin
- Ertapenem
- Erythromycin
- Fosfomycin
- Hetacillin
- Kanamycin
- Linezolid
- Loracarbef
- Meropenem
- Metronidazole
- Nalidixic Acid
- Neomycin
- Nitrofurantoin
- Polymyxin B Sulfate
- Procaine
- Roxithromycin
- Spectinomycin
- Sulfadiazine
- Sulfamethizole
- Sulfamethoxazole
- Sulfanilamide
- Sulfapyridine
- Telithromycin
- Tetracycline
- Tinidazole
- Trimethoprim
- Tobramycin
- Vancomycin
References
- ↑ National Library of Medicine. Antiobiotics. Retrieved on 2007-11-15.
- ↑ Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA (2007). "Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction". Annals of emergency medicine 50 (3): 213-20. DOI:10.1016/j.annemergmed.2007.03.026. PMID 17467120. Research Blogging.
- ↑ Metlay JP, Camargo CA, MacKenzie T, et al (2007). "Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments". Annals of emergency medicine 50 (3): 221-30. DOI:10.1016/j.annemergmed.2007.03.022. PMID 17509729. Research Blogging.
- ↑ Spurling G, Del Mar C, Dooley L, Foxlee R (2007). "Delayed antibiotics for respiratory infections". Cochrane database of systematic reviews (Online) (3): CD004417. DOI:10.1002/14651858.CD004417.pub3. PMID 17636757. Research Blogging.
- Pages using PMID magic links
- CZ Live
- Health Sciences Workgroup
- Chemistry Workgroup
- Infectious Disease Subgroup
- Pharmacology Subgroup
- Biochemistry Subgroup
- Articles written in American English
- Advanced Articles written in American English
- All Content
- Health Sciences Content
- Chemistry Content
- Infectious Disease tag
- Pharmacology tag